In yesterday’s article, I reviewed the immensely regarding information that emerged all through the SSRI antidepressant trials. Sadly, slightly than this information being listened to, it was given a go by the FDA, a sample we now have tragically seen happen with quite a few extremely profitable prescription drugs. In my eyes, three issues stand out concerning the SSRI saga.
The first is that quite a few whistleblowers got here ahead and offered clear proof of precisely how this corruption transpired. The second is that the corruption reached the best ranges of presidency.
The third is that the FDA went to unbelievable lengths to guard the SSRIs, one thing many people wouldn’t imagine could possibly be potential had we not simply witnessed it all through COVID-19.
Note: One of the best points with the SSRIs is how addictive the medication are (stopping them could cause extreme withdrawals that are extremely damaging to the nervous system and typically precipitate violent psychosis). If you’re contemplating stopping them, I strongly suggest working with a well being skilled who’s skilled on this regard.
For those that would not have entry to 1, I compiled an in depth abstract of the best way to safely withdraw from them right here (within the second half of this text).
John Virapen
It is exceedingly uncommon for a pharmaceutical govt to talk out towards their trade (as doing so will completely blacklist them from being employed once more). In flip, the one ones I do know of (in addition to an govt I’ve privately corresponded with) are Peter Rost and John Virapen, each of whom discovered themselves in very distinctive circumstances which enabled and compelled them to talk out towards their trade and disclose the sociopathic habits they noticed inside it.
Note: Rost’s story, together with related accounts from the opposite Pfizer whistleblowers could be discovered on this article and this article.
One of the pharmaceutical executives instantly concerned in acquiring the approval for the unique SSRI antidepressant, Prozac, developed quite a lot of guilt for what he was complicit in as soon as numerous SSRI-linked deaths occurred. In flip, after he was unjustly fired, John Virapen selected to talk out.
Virapen chronicled these occasions in “Side Effects: Death — Confessions of a Pharma Insider.” These included outrageous acts of bribery to get his medication accepted, and photographing physicians with prostitutes offered by Eli Lilly in order that they could possibly be blackmailed into prescribing Lilly’s medication. For these , it is a transient speak that Virapen gave about his experiences. I tremendously admire the actual fact he used candid language slightly than the euphemisms virtually everybody else does:
At the beginning of the saga, Lilly’s senior administration knew Prozac was rubbish and needed to shelve the drug, however since Lilly in dire monetary straits they determined to go all in on the approval of Prozac within the hope it may save the corporate. Prozac, in flip, had initially been proposed as a therapy for weight reduction (as this facet impact of Prozac had been noticed in therapy topics).
However, Lilly in the end concluded (as defined above) it could be a lot simpler to create the phantasm Prozac handled “melancholy” after which get a post-marketing approval for the therapy of weight reduction.
As Prozac took off, it turned clear that melancholy was a significantly better market, and the weight problems facet was forgotten. Lilly then used a standard trade tactic and labored tirelessly to broaden the definition of melancholy so that everybody may grow to be eligible for the drug and aggressively marketed this want for happiness to the general public, earlier than lengthy, reworking melancholy from a uncommon to a standard one.
Unfortunately, whereas the advertising machine had no difficulties creating a requirement for Prozac, the preliminary medical trial information made it abundantly clear that the primary SSRI, Prozac, was harmful and ineffective. Lilly settled on the technique of acquiring regulatory approval in Sweden, and utilizing this approval as a precedent to acquire approval in different international locations.
Virapen was assigned to this job and informed by his superiors that if he failed, his profession was over. Virapen, sadly, found that at any time when he offered Lilly’s medical trial information to consultants, they laughed and had hassle believing he was truly searching for regulatory approval as Prozac’s trial information was simply that dangerous.
Sweden (following their regulatory procedures) elected to permit an out of doors unbiased knowledgeable to make the ultimate dedication on whether or not Prozac needs to be accepted or not. The id of this knowledgeable witness was hid, however Virapen was in a position to decide that it was Anders Forsman, a forensic psychiatrist and member of the authorized council on the Swedish National Board of Health.
After assembly with Virapen, Forsman proposed an untraceable bribe. Then, upon receiving cost, wrote a glowing letter in assist of Prozac, absolutely reversing his earlier place (he had ridiculed it simply two weeks earlier than) and guided Virapen by re-writing the trial to hide the 5 tried (4 of which had been profitable) SSRI suicides in it.
Forsman’s “knowledgeable” opinion resulted in Prozac being partially accepted and formally priced for reimbursement in Sweden, which was then used as a precedent to promote it all over the world at that very same profitable value.
Note: After leaving Lilly, Virapen tried to have Forsman prosecuted for bribery. Despite the chairman for the Institute towards Bribery submitting a report back to the Department of Justice affirming bribery had certainly occurred, Forsman (who repeatedly lied all through the method) was not prosecuted as a result of he was not an official worker of the company. Forsman in flip was allowed to proceed his skilled profession and was employed by the state lengthy after the investigation ended.
Virapen famous that in this time, German drug regulators who had clearly and unambiguously acknowledged that Prozac was “completely unsuitable for the therapy of melancholy” all of a sudden reversed their place, main Virapen to suspect that related under-the-table exercise should have occurred in Germany.
David Healey, a health care provider and director of the North Wales School of psychological medication, likewise concluded that the German approval was as a result of “unorthodox lobbying strategies exercised on unbiased members of the regulatory authorities.”
Note: A key purpose why the German regulators initially refused to approve Prozac was as a result of the particular standards used for figuring out an enchancment in melancholy was extremely subjective and the profit was solely being reported by the trial psychiatrists however not the individuals themselves.
Not lengthy after saving Eli Lilly, Virapen was fired. Virapen believes he was fired as a result of he was a person of colour in an in any other case Caucasian firm (he was informed this by his supervisor).
Peter Gøtzsche, a number one knowledgeable in pharmaceutical analysis fraud, alternatively, attributed this to typical organized crime ways the place Lilly sought to hide their criminality by firing Virapen and his two assistants (as instantly after their abrupt termination, none of them had been permitted to entry their workplaces, and thus couldn’t get hold of any of the recordsdata that proved that they’d bribed Forsman).
In brief, given how horrendous the information supporting their security and efficacy was, you have to be questioning how the SSRIs made it by the regulatory approval course of.
George H.W. Bush
There is a number of darkish historical past to the Bush household. The Bush dynasty was based by Prescott Bush, who constructed his household fortune by collaborating with the Nazis instantly towards the needs of the U.S. authorities (The Guardian, for instance, confirms it right here).
His son, George H.W. Bush had the distinctive accomplishment of being the one CIA chief to later grow to be president, and through his transient tenure there was chargeable for quite a few crimes towards humanity in South America. After leaving the CIA as soon as Carter turned president, Bush (senior) served as a board member for Eli Lilly.
He then joined the Reagan Administration as Vice President, the place he helped to push by the catastrophic determination for the FDA to approve aspartame for shopper use (aspartame was so harmful even the FDA didn’t need to approve it). After succeeding Ronald Reagan as President, Bush selected Dan Quayle as his Vice President:
“In Talking Back to Prozac (1994), I identified that Prozac was accepted beneath the primary Bush administration and that George Bush had been a member of the board of administrators of Eli Lilly, the producer of Prozac. I additionally identified that Vice President Dan Quayle was from Indiana, the house state and worldwide headquarters for Eli Lilly.
At the time the FDA was approving Prozac, Quayle employed former Eli Lilly personnel on his personal employees, and Quayle had appreciable leverage over the FDA because the chair of a particular committee that was investigating its operations.
I questioned whether or not the FDA may need rejected Prozac and that the whole SSRI onslaught would possibly by no means have gotten began if the president and vice chairman of the United States had not been so intently affiliated with Eli Lilly.”
Bush’s son, President George W. Bush likewise adopted in his father’s footsteps and appointed Eli Lilly executives to senior positions inside his administration. In truth, he even inserted a provision into the Patriot Act to exempt vaccine producers, together with Eli Lilly, from legal responsibility for thimerosal (Mercury) inside vaccinations.
In brief, Bush profoundly modified the FDA’s regulatory conduct. Consider this instance shared by John Virapen that occurred a couple of years earlier than Bush turned president. In 1980, Eli Lilly utilized for the approval of benoxaprofen, and aggressively promoted this new blockbuster medicine.
Not lengthy after being accepted, in 1982, benoxaprofen was taken off the market after being linked to a small variety of deaths, and Eli Lilly underwent a prolonged investigation carried out by the Justice Department, the place it was concluded that Lilly deliberately coated up the deaths attributable to their drug. Benoxaprofen is banned, however nothing remotely related has been completed for the SSRIs.
SSRIs and the FDA
The FDA’s therapy of the SSRIs is among the solely cases I do know of, the place, just like the COVID vaccines, the company has not solely ignored, however actively tried to hide a horrific variety of opposed occasions for a pharmaceutical regardless of receiving widespread protest from the general public. This was more than likely closely influenced by the Bush Administration being in mattress with Eli Lilly.
As such, it’s insightful to see how this has performed out over many years, as we ponder how the FDA will deal with the COVID vaccines and what we have to do to deal with this mess. First, think about the FDA’s habits when Bush was not but the president:
“Initially, the FDA was skeptical and famous severe flaws in Lilly’s trials. An FDA officer wrote in 1984 that sufferers who didn’t do properly after two weeks had their blinding damaged, and in the event that they had been on placebo, they had been switched to fluoxetine (leading to six weeks of fluoxetine being in comparison with two weeks on placebo).
An FDA evaluation additionally found that 25% of the sufferers had taken a further drug, and when the FDA in 1985 eliminated sufferers on different medication from Lilly’s trials, there was no vital impact of fluoxetine.
By including benzodiazepines, Lilly broke the principles for its trials however didn’t inform the FDA, and when the FDA later realized about it, the company permitted it and thereby broke its personal guidelines. The public and the docs had been by no means knowledgeable about this ruse.”
Prozac was in the end accepted in December 1987, at which level 3 of the 4 research that this approval was based mostly upon used benzodiazepines to hide the agitating or psychotic syndromes created by the SSRI medication.
Note: A very good case could be made that lots of the advantages attributed to SSRIs truly had been because of the benzodiazepines that had been used concurrently with them.
Once Prozac entered the market in 1988, opposed occasion experiences started to build up, and by 1991, Prozac had one of many highest charges of opposed occasions ever reported to FAERS (much like VAERS however for different pharmaceutical accidents).
As there was much less regulatory seize on the time, these pink flags had been ample to convene a Congressional listening to on the SSRIs (whereas immediately, apart from one held a month in the past by Congresswoman Marjorie Taylor Greene, this nonetheless has not occurred for the COVID-19 vaccines).
Note: In the primary 9 years, the FDA obtained 39,000 opposed occasion experiences, excess of for another drug. In these, there have been hundreds of suicides (e.g., by 1999 over 2000 Prozac suicides had been reported), horrendous crimes, hostility, psychoses, confusion, irregular considering, convulsions, amnesia and sexual dysfunction.
A 1991 FDA listening to was convened the place many witnesses informed tales about out-of-character suicides and homicides. The advisory committee members, lots of whom had monetary ties to pharmaceutical firms producing SSRIs, ignored these experiences and unanimously rejected the next proposal:
“There is credible proof to assist a conclusion that antidepressant medication trigger the emergence and/or the intensification of suicidality and/or different violent behaviors.”
Note: Internal Lilly paperwork revealed that the FDA had already been working with Lilly on the suicide concern (and that beforehand Lilly had disclosed to German regulators that Prozac doubled the chance of suicide in comparison with placebo). However, on the assembly, the chair of the FDA committee interrupted an out of doors knowledgeable who tried to share this, leading to a lot of the presentation being carried out by Lilly workers who had been in a position to current Lilly’s narrative to everybody).
Similarly, on the time this listening to occurred, the FDA’s personal workers had been elevating issues concerning the security of Prozac. Furthermore, a later obtained doc confirmed that the FDA knew that the suicide fee on Prozac was 0.52% (vs. 0.18% on placebo), and that in Pfizer’s Zoloft submission (which reported a 26% lower in suicide makes an attempt), when the FDA counted the deaths appropriately, there was truly a 29% enhance in them.
Sadly, shopping for out “knowledgeable” committees is a normal trade follow. To additional illustrate the illegitimacy of those committees (who’re entrusted to determine a lot of public coverage), think about this report from Kim Witczak, a citizen activist who was in a position be appointed to one in all them:
“Fast ahead, after Pfizer settled the Chantix lawsuits Pfizer went to the FDA to ask to have the black field neuropsychiatric warning faraway from their drug label. By this time, I used to be the Consumer Representative on the FDA Psychopharmacologic Drugs Advisory Committee.
We had been going to evaluation Pfizer’s new EAGLE research. I used to be actually wanting ahead to being a part of this committee and had many inquiries to ask concerning the security, the lawsuits, the inner firm paperwork found and reviewed by consultants, and most significantly, the victims.
After all, Pfizer simply settled the lawsuits for nearly $300 million and silenced everybody. One would suppose the FDA committee would need to have all data together with what was found in lawsuits involving 2700+ victims earlier than making any choices to take away the warnings.
A couple of days earlier than the FDA Advisory Committee, I obtained an e mail from the FDA that they needed to speak with me concerning the upcoming advisory committee assembly. Someone (cough Pfizer) introduced it to their consideration that I had an “mental bias” and shouldn’t serve on the committee.
The roomful of FDA staffers informed me that I used to be being recused from serving on this assembly. I informed them in the event that they suppose security is an mental bias (or a viewpoint), I’ll at all times have one.
Much to their shock, I stated I might nonetheless like to deal with the committee and converse through the open public listening to. I ended up flying out a couple of days later alone time and dime to verify my feedback and questions had been requested despite the fact that they wouldn’t be a part of the official public file of this assembly.
Ultimately, in an unprecedented transfer, the FDA eliminated this severe black field warning that concerned violence, hallucinations, suicide, and different psychiatric unintended effects. To at the present time, this story has by no means actually been informed by the media. These unintended effects didn’t all of a sudden go away. Just the FDA black field warnings.”
As detailed above, lawsuits towards SSRI producers like Lilly have repeatedly revealed these firms intentionally hid the opposed occasions that occurred of their trials. Similarly, Lilly additionally selected to commit fraud by illegally failing to report 76 of 97 circumstances of suicidality from Prozac in a post-marketing surveillance research it submitted to the FDA.
Furthermore, Lilly additionally didn’t report that, Cymbalta, an SNRI steadily marketed for treating continual ache, was discovered to trigger extreme withdrawals as soon as discontinued in half of those that had obtained it for no less than 8 weeks. In flip, within the first quarter of 2012, extra experiences had been submitted to the FDA on severe drug withdrawal results for Cymbalta than for another recurrently monitored drug, together with two opioids.
Note: Paxil can be infamous for being extremely addictive (e.g., of their unique license utility they acknowledged 30% of trial topics skilled withdrawals), however for the primary ten years it was available on the market, GSK adamantly claimed it was not addictive. Eventually (in 2001) the WHO acknowledged Paxil had the best withdrawal problems with any SSRI available on the market (which was adopted by a warning from the FDA in 2002).
GSK in flip lastly “admitted” this by revising its prescribing directions to state the chance of withdrawals was not 0.2% however as a substitute 25% (a 125 fold enhance).
Organized Cover-Ups
One of essentially the most blatant examples of how far the FDA will go to guard the trade occurred in 2003, when whereas analyzing a medical trial for giving Paxil to youngsters, the FDA observed that extra episodes of “emotional lability” (speedy, usually exaggerated adjustments in temper) had been reported in youngsters on Paxil than these on a placebo.
The FDA determined to research what the precise symptom Paxil’s producer was concealing behind this label, and was knowledgeable most circumstances referred to suicidality. One of the FDA’s security officers, Andrew Mosholder, a toddler psychiatrist, additional investigated this concern and concluded that 22 research confirmed that youngsters given antidepressants had been almost twice as more likely to grow to be suicidal as these given placebos.
His superiors on the FDA who had not too long ago hidden Paxil’s tendency to trigger suicidality in youngsters predictably disputed his report, and didn’t enable it to be launched to the general public or offered at an advisory assembly. A yr later in 2004, the report was leaked, and in a really telling transfer, the FDA selected to conduct a felony investigation of the leak slightly than tackle the clear security issues it had raised.
Kim Witczak spearheaded many various initiatives towards the SSRIs. For instance, she filed a wrongful loss of life, failure to warn lawsuit towards Pfizer (which Pfizer responded to by sending investigators round her neighborhood to dig up grime on her). Her lawsuit was in a position to get hold of many essential paperwork from Pfizer proving that they knew how harmful their SSRI was (together with the identical out-of-body experiences which her husband had had earlier than killing himself).
Her lawsuit finally offered the ammunition to get a black field warning (simply seen red-alerts the FDA sometimes mandates for prescription drugs) positioned on the SSRIs.
Note: Documents confirmed that Lilly initially deliberate to have a warning for Prozac inflicting psychosis within the USA bundle insert, however in the end solely did so in Germany, as their regulators, in contrast to the FDA, required Lilly to insert this warning.
Because of her efforts, just like the earlier instance confirmed, Witczak was supplied with a direct view into the corruption throughout the FDA. For instance, that is how they addressed the “downside” that lawsuits towards the SSRI producers had been inflicting their confidential paperwork (detailing the precise harms of the medication) to be launched:
“Pfizer used the FDA to intervene in Baum Hedlund’s civil lawsuits. It was found that Pfizer paid trade protection lawyer Dan Troy $300k for some authorized work shortly earlier than he was appointed FDA Chief Counsel by President Bush. In his new position on the FDA, Dan Troy was the mastermind behind the FDA preemption amicus “good friend of the court docket” transient intervening on behalf of pharmaceutical firms in civil lawsuits.
The transient [falsely] argued that as a result of drug was FDA accepted, the lawsuits had been “preempted” and needs to be dismissed.
The transient [falsely] claimed even when an organization needed to warn shoppers, the FDA wouldn’t allow them to replace their warning label if the FDA didn’t agree. Many Zoloft suicide lawsuits had been tossed out by judges who believed the FDA was last authority on the drug label. Pfizer even tried arguing the FDA preemption transient in my lawsuit. Not as soon as, however twice.
Federal Chief Justice James Rosenbaum disagreed with Pfizer and allowed my lawsuit to proceed.
We labored with NY Representative Maurice Hinchey to assist expose the $300k Dan Troy obtained from Pfizer. Ultimately Dan Troy resigned his FDA Chief Counsel publish however not earlier than injury was completed. He in the end went again to work for personal trade together with turning into international Chief Counsel at GlaxoSmithKline, the maker of Paxil, one other SSRI.”
Sadly, paying off regulators (e.g., by giving them comfortable jobs of the pharmaceutical trade) is quite common (the follow is called the “revolving door”). For occasion, lots of the authors of presidency research (e.g., FDA workers) who questionably decided the SSRIs had been “secure and efficient” had been additionally paid off by the SSRI producers.
In 2004, because of the mounting political stress, the FDA lastly launched a black field warning linking SSRIs to elevated suicidality in youngsters. Despite realizing about this downside lengthy earlier than the SSRIs got here to market, it took over 20 years for the FDA to supply this important warning.
More importantly, this solely occurred after huge public stress, numerous lawsuits proving these results had been intentionally hid by the producers, public hearings, and leaked experiences publicly shaming the FDA.
Note: In 2006, the warning was prolonged to everybody beneath the age of 25. As this lower off was fully arbitrary (lots of the SSRI suicides occurred in a lot older people) a big press convention was organized the day beforehand so these believing it wanted to be utilized to all ages may have the time to talk the FDA wouldn’t allow them to have throughout its listening to.
Although their motion didn’t persuade the FDA to vary course, subsequent yr in 2006, the FDA did and utilized that warning to all ages teams.
By 1990, the general public was demanding for the FDA to find out if SSRIs had been linked to elevated suicidality. As the proof proving this was unambiguous, the FDA intentionally prevented publishing a report on this matter. Sixteen years later, shortly after the FDA was uncovered for suppressing the hyperlink between suicidality in youngsters and SSRIs, the FDA lastly printed a meta-analysis addressing this query.
The 2006 meta-analysis encompassed 372 placebo-controlled trials of SSRIs (and associated medication) involving 100,000 sufferers, and confirmed that as much as the age of 40, SSRIs elevated suicidal habits, whereas in older sufferers SSRIs decreased this danger.
Note: A typical tactic within the pharmaceutical trade is to hyper-focus on one particular set of unintended effects in order that the opposite unintended effects could be coated up.
For instance, from evaluating the incidences of blood clots I hear about relative to the share of people that selected the J&J vaccine, I’m comparatively sure that the mRNA vaccines usually tend to trigger blood clots than J&J’s, however at any time when this matter is raised, individuals default to believing solely J&J could cause blood clots because it was linked to a couple circumstances of central venous thrombosis and there was a quick interval the place the vaccine was suspended by the FDA to “assess” this danger.
I think that the FDA’s long-delayed meta-analysis and the black field warning had been a direct response to the leaked report proving an indeniable hyperlink between SSRIs and adolescent suicidality that was produced to defend the opposite unintended effects from scrutiny. Sadly, these warnings have completed little or no to curb the utilization of those medication, as evidenced by how giant their market has grow to be.
Rather they served as a technique to defend that market as they each had been a substitute for pulling the medication (which is what ought to have occurred) and downplayed the unintended effects as a lot as potential (e.g., borrowing from the trade’s playbook, “irregular ideas” turned irregular desires).
Furthermore, the FDA’s meta-analysis virtually actually additionally understated the chance. For instance, the FDA gave the research they analyzed a free go on the number of design flaws that made it straightforward to hide their opposed occasions. In truth, the FDA reached out to lots of the SSRI producers and requested them to adjudicate (take away) probably suicide-related opposed occasions of their trials as they noticed match and ship these outcomes to the FDA.
When analyzing the 2006 meta-analysis, Gøtzsche discovered quite a few different indicators of deliberate fraud by the FDA. For instance, in lots of circumstances (usually as a result of information revealed from litigation), a single research throughout the meta-analysis was proven to comprise extra circumstances of suicide from an SSRI than the 5 suicides the FDA claimed had occurred all through all 372 of its research.
From extensively reviewing all the information, Peter Gøtzsche, reached the general conclusion that there are more likely to have been 15 instances extra suicides on antidepressant medication than reported by the FDA in its 2006 meta-analysis.
Note: In 2006, 35 million was spent by American’s National Institutes of Mental Health to conduct the STAR*D research, which assessed if SSRIs cured “therapy resistant” melancholy (making it the biggest research on SSRI efficacy ever carried out) and was designed to evaluate typical sufferers in actual life situations (though the care they obtained was probably higher than what’s seen in medical follow).
It discovered 3% or much less of topics had their melancholy cured (with it not remitting for the yr of remark throughout the trial). However, the NIMH repeatedly acknowledged “about 70% of those that didn’t withdraw from the research turned symptom-free,” considerably exaggerated the enhancements within the sufferers, and that SSRI therapy was far simpler that placebo, regardless of no placebos getting used within the trial.
In my private opinion, when your outcomes are off by an order of magnitude, this may solely happen by deliberate fraud, one thing many people have regrettably come to appreciate has occurred at each the CDC and the FDA all through the COVID-19 vaccination marketing campaign.
As it so occurred, by 2013, the FDA worker in command of the 2006 meta-analysis had fully transitioned to the non-public sector and had made a consulting agency devoted to serving to psychiatric medication sail by the FDA.
Note: A wide range of different giant research have used related strategies to hide the hazards of the SSRIs. Since I can’t cowl all of them right here, I selected to give attention to ones carried out by the US authorities.
The Big Lie
When Hitler wrote Mein Kampf in 1925, he described how individuals could possibly be induced to imagine a colossal a lie as a result of they’d not imagine that somebody “may have the impudence to distort the reality so infamously.” While he initially used this concept to assault others (e.g., the Jews), earlier than lengthy he absolutely adopted it, permitting the Nazi regime to grow to be one of the crucial highly effective forces of propaganda in historical past.
Many others have additionally used this strategy. For instance (as mentioned in a latest article), for many years, US well being authorities ({and professional} medical associations) have repeated the mantra that their vaccine is “secure and efficient” whereas concurrently suppressing all proof on the contrary (e.g., from their very own scientists).
This in flip has resulted in quite a few disastrous vaccines (which everybody knew had been dangerous) being pushed onto the market and never being taken off till a major quantity of accidents had occurred. With the SSRIs, we see an analogous diploma of audacity, as time and time once more the SSRI advocates will insist their medication are secure and efficient regardless of all proof on the contrary. For instance:
“In 2014, the medical director on the Norwegian drug company, Steinar Madsen, stated at a gathering that antidepressants work for 50-60% of the sufferers. I [Peter Gøtzche] replied that his assertion illustrated why we can’t belief our drug regulators and reminded him that the FDA had discovered of their evaluation of 100,000 sufferers that antidepressants labored for under 10% of the sufferers.
Throughout the Nineteen Nineties, whereas swearing publicly that fluoxetine didn’t enhance the chance of suicide or violence, Lilly quietly settled lawsuits out of court docket and saved the incriminating proof hidden by acquiring court docket orders to seal the paperwork.
[In 2011 the CEO of a company that sold five antidepressants], claimed in a radio programme that SSRIs scale back suicides in youngsters and adolescents. When the shocked reporter requested him why the bundle inserts warned towards suicide makes an attempt, additionally for Lundbeck’s medication, he replied that he anticipated the leaflets could be modified by the authorities!
The radio interview happened whereas Lundbeck’s US companion, Forest Laboratories, was negotiating compensation with 54 households whose youngsters had dedicated or tried suicide beneath the affect of Lundbeck’s antidepressant medication.
[BBC Journalist] Shelley Joffre, confirmed that the GSK spokesperson, Dr Alastair Benbow, lied in entrance of a operating digital camera. He denied, for instance, that paroxetine may trigger suicidality or self-harm whereas he despatched information to the drug regulator one month later that confirmed precisely this, and which instantly led to a ban on utilizing paroxetine in youngsters.”
Note: The UK drug regulators additionally lied to the general public to cowl for GSK (which is predicated within the UK) by stating that the invention Paxil precipitated these suicides was fully new to the corporate (whereas paperwork confirmed it had in actual fact identified about it for no less than eight years). Furthermore, when US senator Charles Grassley later requested GSK for a way lengthy the corporate had identified that paroxetine will increase the suicide danger, GSK repeated this lie, claiming GSK had not detected the chance till 2006.
Given their willingness to blatantly lie, even to a US Senator, it ought to come as no shock these firms concocted elaborate methods to silence their critics. For instance, GSK has publicly acknowledged:
“Major depressive dysfunction is a doubtlessly very severe sickness related to substantial morbidity, mortality, suicidal ideation, suicide makes an attempt and accomplished suicide. Unwarranted conclusions concerning the use and danger of antidepressants, together with paroxetine, do a disservice to sufferers and physicians.”
Many psychiatrists (particularly these being paid off by the pharmaceutical trade) in flip have used related arguments to silence all criticisms of their medication. Sadly these ways aren’t distinctive to the psychiatric trade. For instance, in a earlier article I mentioned the numerous risks (and full lack of profit) from statins.
In flip, at any time when statins are questioned, slightly that defend them, cardiologists will usually insist you’re “killing sufferers” by scaring them away from the medication, and this argument has been efficiently towards each physicians and information applications which questioned statins. In flip, as you would possibly guess, that tactic has additionally been used towards critics of the SSRIs.
“In New Zealand, psychiatrists and suicidologists managed to persuade the federal government [with very weak evidence] that publishing data on suicides causes copycat suicide, which in flip made it a felony offense for victims or the media to publicly talk about SSRI suicides.”
Likewise, this similar playbook has been used towards critics of a controversial vaccine. Sadly, since there had been quite a few trial runs with different lethal merchandise, by the point COVID-19 occurred, the “harmful misinformation” playbook had been developed, and that label was instantly plastered onto anybody who questioned any a part of the pandemic response (e.g., the lockdowns, the suppression of early therapy or the COVID-19 vaccines).
This in flip set the stage for the place it someway turned acceptable to argue individuals needs to be compelled to vaccinate towards their will regardless of a major quantity of proof (and public opinion) current that argued towards vaccinating. In some ways, this isn’t that totally different from how psychiatric medicine mandates are sometimes pushed upon sufferers who (as a result of their unintended effects) merely are not looking for them.
Note: There are many unhappy tales of this — together with quite a few ones the place the courts supported the psychiatric mandate irrespective of how a lot work was completed to overturn them.
Conclusion
In my eyes, one of the crucial vital issues to think about on this article is simply how many individuals are taking SSRIs, and by extension, simply what number of accidents the chances I offered on this article translate to. Whenever a drug is being thought-about for approval, one of many main issues by the regulator was the overall anticipated harms instructed by the preliminary information — but as we will see each within the SSRI saga and all through COVID-19, that precept has merely been discarded.
As I ponder how issues may have gotten this manner and the way symbiotic the connection has grow to be between the pharmaceutical firms and the drug regulators, I’m reminded of this iconic scene from Idiocracy:
The saddest factor concerning the SSRI saga is that as inexcusable because it was, issues had been a lot much less corrupt then than they’re now, particularly throughout the federal authorities. At the time that the general public challenged the SSRIs, the media would air tales important of the malfeasance throughout the federal authorities and lawsuits may compel the pharmaceutical firms to reveal the harms they had been hiding from the general public, and Congress was prepared to research.
Now, all of the vaccine producers have virtually full safety from legal responsibility and apart from a couple of commentators on Fox News, nobody a lot as dares to query the vaccines (or another pharmaceutical for that matter). One remark Kim made on our unhappy state of affairs actually caught with me:
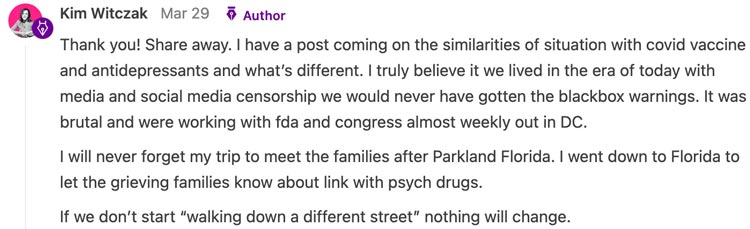
Note: Renowned journalist Sharyl Attkisson has made a wonderful case the prolific censorship we now have grow to be accustomed to started through the Obama presidency.
My hope is that the hurt of the COVID-19 vaccines is so egregious and unambiguous, and extra importantly, has affected so many individuals, that it’ll immediate sufficient public outcry to repair or no less than enhance this systemic corruption.
In this collection, I’ve tried for instance how the gross malfeasance that allowed the SSRIs to be introduced onto the market and saved there regardless of numerous pink flags telling the FDA the medication weren’t secure. Overcoming the stress to take these medication off the market in flip required some huge cash to be behind these medication.
In the ultimate a part of this collection, we’ll discover how the SSRI trade satisfied the world everybody wanted their (sometimes nugatory) drugs (whereas concurrently inflicting many efficient SSRI therapies to be dismissed and forgotten). Much of our tradition is formed by the pharmaceutical trade manufacturers illnesses and I imagine the ways they use have to be acknowledged so our society stops falling sufferer to them.
I thank every of you for studying this collection and serving to carry consideration to this tragedy as many individuals I’m near have been.
A Note From Dr. Mercola About the Author
A Midwestern Doctor (AMD) is a board-certified doctor within the Midwest and a longtime reader of Mercola.com. I admire his distinctive perception on a variety of matters and I’m grateful to share them. I additionally respect his need to stay nameless as he’s nonetheless on the entrance strains treating sufferers. To discover extra of AMD’s work, you’ll want to try The Forgotten Side of Medicine on Substack.

