[ad_1]
In a latest examine printed within the journal PNAS, researchers investigated how large-scale livestock rearing might consequence within the emergence and transmission of novel, probably zoonotic pathogens. They mixed a number of traces of proof from molecular courting, comparative genomic analyses, and phylogeography to look at how the rearing of pigs allowed for novel Streptococcus suis lineages, a few of that are able to zoonotic spillover. Their findings reveal how pathogens, together with S. suis, adapt to take advantage of substantial adjustments of their host inhabitants sizes and the way this, in flip, can not directly contribute to the emergence of novel, probably pandemic-scale, and zoonotic, extremely pathogenic strains of hitherto benign microbes.
 Study: The emergence and diversification of a zoonotic pathogen from inside the microbiota of intensively farmed pigs. Image Credit: Dusan Petkovic / Shutterstock
Study: The emergence and diversification of a zoonotic pathogen from inside the microbiota of intensively farmed pigs. Image Credit: Dusan Petkovic / Shutterstock
Livestock rearing and its results on pathogens
Over the previous few centuries, the human inhabitants explosion and its related want for livestock as meals and labor to help within the agricultural business has resulted within the international upscaling of livestock rearing. The agricultural suggestions loop of intensive farming methods allows bigger livestock populations, which in flip calls for elevated crop manufacturing. This has resulted in livestock populations now exceeding the mixed populations of people and wild animals.
These practices, together with the long-distance transport of reared animals, have contributed to low-genetic variety and high-density livestock. This presents a perfect recipe for outbreaks of pathogens able to wiping out thousands and thousands of livestock with out the genetic capability for resistance, which, when transported, can infect not solely different livestock populations but in addition wild populations of the identical or related species.
Alarmingly, this cocktail of occasions is hypothesized to advertise the emergence of novel zoonotic pathogens, arising from pathogens leaping to new hosts and mutations in beforehand benign microbiota beforehand related to reared animals.
“This route to pathogen emergence may be particularly important in intensive farming systems, where large population size and high population density may select for traits associated with pathogenicity, while biosecurity reduces the risk of novel pathogens entering the population.”
Streptococcus suis is a ubiquitous microbiota element of the higher respiratory tract of pigs. Previously benign, intensive rearing of pigs within the 19th and 20th centuries, the microbe has been noticed to adapt to a extra pathogenic way of life. In 1954, the micro organism was implicated in widespread illness in pigs, and right now presents one of the vital widespread illnesses in piglets. Alarmingly, gathered mutations have allowed the micro organism to zoonotically spill over to people, related to meningitis, arthritis, endocarditis, and septicemia, with sudden loss of life in each human and porcine hosts.
Following the primary human S. suis-related mortality in 1968, the micro organism has since led to giant outbreaks in China and presents one of many main causes of grownup septicemia and meningitis throughout Southeast Asia.
“Difficulties in identifying the determinants of pathogenicity in S. suis have been attributed to its complex pathogenesis and high level of genetic diversity. Few studies have considered virulence factors in strains other than ST 1, which is responsible for most cases of S. suis disease in both pigs and humans worldwide.”
About the examine
In the current examine, researchers investigated the associations between intensive pig rearing, the emergence of novel S. suis lineages, and their potential for zoonotic spillover. They carried out a inhabitants genomic evaluation of over 3,000 bacterial samples derived from tonsil and nasal swabs from pigs and wild boar. They moreover collected contaminated blood from people and pigs affected by S. suis illness throughout North America, Europe, Asia, and Australia. They aimed to elucidate the emergence, geographic unfold, and diploma of diversification of pathogenic lineages of the micro organism.
The examine dataset comprised 3,070 genomic isolates of S. suis samples derived from beforehand current printed knowledge and picked up and sequenced as part of this undertaking. This included 29 printed reference genomes and assortment isolates from 15 nations unfold throughout the 5 continents above. Sampling was carried out between 2014 and 2018. Isolates have been processed by the Illunima complete genome HiSeq 25000 sequencing pipeline, which was then used to construct a genomic library of isolates. Raw sequences have been quality-checked, cleaned, and used to generate de novo assemblies for polymorphism evaluations.
The pipeline described by Athey et al. was used for serotyping and sequencing-typing analyses. Generated genomes have been subsequently annotated to establish homologous genes and analyze pathogenicity-associated genomic islands. The PopPunk software program was then used to establish divergent genomes and classify them into lineages. The six most typical lineages thus recognized have been examined for temporal indicators utilizing a regression of root-to-tip distances towards the yr of isolate sampling. Finally, ancestral state reconstructions have been used to deduce the geographic unfold of recognized lineages.
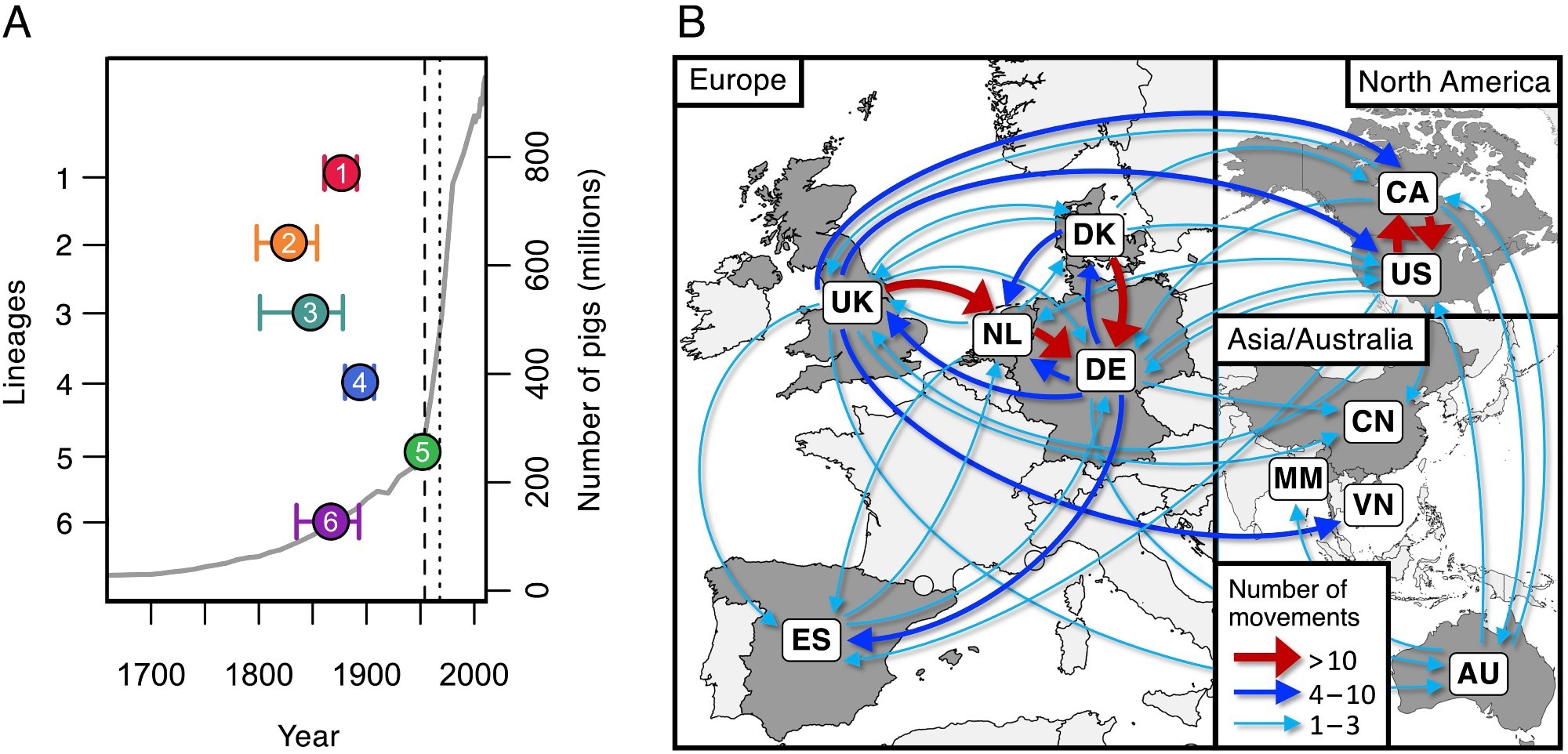 Dates of emergence and paths of between-country transmission for the six most typical pathogenic lineages. (A) Estimates of the dates of the newest widespread ancestors of the six most typical pathogenic lineages (coloured factors) towards an estimate of the worldwide variety of pigs (grey line). The vertical dashed line exhibits the date of the primary reported case of S. suis illness in pigs (1954), and the dotted line exhibits the primary reported human case (1968). (B) Map exhibiting inferred routes of transmission of those six pathogenic lineages between the nations in our assortment. Arrows symbolize routes with no less than one inferred transmission occasion. Routes with greater than ten inferred transmission occasions are proven in pink, these with greater than three in blue, and people with one to a few in turquoise.
Dates of emergence and paths of between-country transmission for the six most typical pathogenic lineages. (A) Estimates of the dates of the newest widespread ancestors of the six most typical pathogenic lineages (coloured factors) towards an estimate of the worldwide variety of pigs (grey line). The vertical dashed line exhibits the date of the primary reported case of S. suis illness in pigs (1954), and the dotted line exhibits the primary reported human case (1968). (B) Map exhibiting inferred routes of transmission of those six pathogenic lineages between the nations in our assortment. Arrows symbolize routes with no less than one inferred transmission occasion. Routes with greater than ten inferred transmission occasions are proven in pink, these with greater than three in blue, and people with one to a few in turquoise.
Study findings
Study findings revealed that over the previous 200 years, rearing porcine populations have elevated by over 200-fold, with the utmost improve throughout the latter half of the 20th century. These will increase have resulted within the admittedly gradual but regarding emergence of over 10 lineages with extremely pathogenic life histories. The excessive density of reared pigs, normally antibiotic-treated, has led to numerous, antibiotic-resistance lineages of S. suis presenting important management challenges.
Analyses of samples from Spain reveal that pigs, each reared and wild boar, are hosts to S. suis strains which can be extremely genetically numerous, suggesting that the affiliation between the microbe and its host has been long-standing. Genetic courting analyses, nonetheless, revealed that all the six most typical pathogenic S. suis strains emerged throughout the 19th and 20th centuries, corresponding with the unprecedented improve in host rearing.
“The conclusion that these dates reflect an ecological shift toward pathogenicity in at least some of these lineages is supported by evidence that they coincided with the acquisition of a pathogenicity-associated genomic island (Island 3). It is further supported by patterns of genome reduction in each of the pathogenic lineages. In comparisons across bacterial species, it has been shown that bacterial pathogenicity is broadly associated with smaller genomes and fewer genes.”
Analyses of the metabolic capacities of pathogenic lineages revealed that no less than two recognized genomic islands had considerably upped their capabilities for within-host progress, with all six islands depicting elevated metabolic actions over their extra benign counterparts. In different micro organism research, each within-host progress and metabolic price have been linked to elevated virulence, suggesting a development in S. suis adapting to a extra virulent life historical past with better potential for zoonotic spillover.
“Pathogenic lineages may be better able to exploit particular regions of the tonsil than commensal lineages and vice versa, thereby reducing within-host competition. This could lead to segregation of these populations and reduced gene flow between them, which could in turn lead to the genome reduction in more pathogenic lineages due to fewer opportunities for gene acquisition from more diverse commensal lineages.”
Finally, analyses revealed that present S. suis strains depict a excessive price of unfold, able to quickly infecting a whole farm of pigs with the addition of 1 or a number of contaminated people. The large-scale transport of livestock thereby presents a further drawback: earlier endemic virulent strains being transmitted throughout nations and even continents, able to infecting novel host populations with little to no innate resistance towards them.
“Our results provide a framework for understanding the genomic diversity in S. suis and its association with pathogenicity. This is likely to be of widespread use in S. suis research and in informing strategies for controlling the burden of this disease on pig farming and human health. As our collection spans only a small proportion of the countries that farm pigs globally, further sampling from a broader range countries and more extensive sampling within countries, particularly those with large and growing pig populations, is needed to investigate the existence of additional pathogenic lineages that are geographically restricted or have recently emerged.”
[ad_2]
