In July 2018, FDA launched the Biosimilars Action Plan (BAP), which outlined FDA’s strategy for increasing entry to biosimilars for the American public. The plan targeted on 4 key areas:
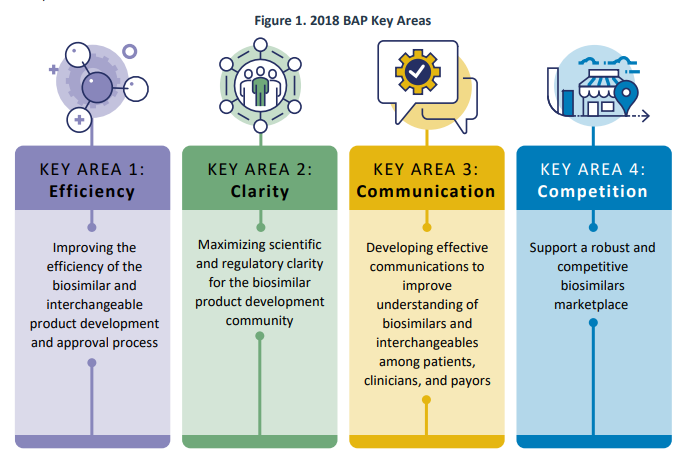
A current FDA report critiques a few of their accomplishments since then. Most of those efforts revolve round steering paperwork, further workers, schooling merchandise and web sites, public hearings and rules (i.e., proposed/remaining guidelines). FDA additionally added new information sources together with publishing a modernized model of the Purple Book in February 2020. FDA additionally collaborated with different companies reminiscent of FTC, and produced a joint assertion and held a workshop in March 2020, entitled: “Public Workshop: FDA/FTC Workshop on a Competitive Marketplace for Biosimilars.” Some different key actions are listed beneath.

The full report is right here.
