[ad_1]
I simply got here throughout a pleasant overview article titled The monetary ecosystem of pharmaceutical R&D. The white paper was developed for the Netherlands authorities and supplies an summary of the drug improvement course of and the way (and by whom) it’s financed. The white paper solutions the next questions:
What do completely different gamers do throughout the drug improvement ecosystem?
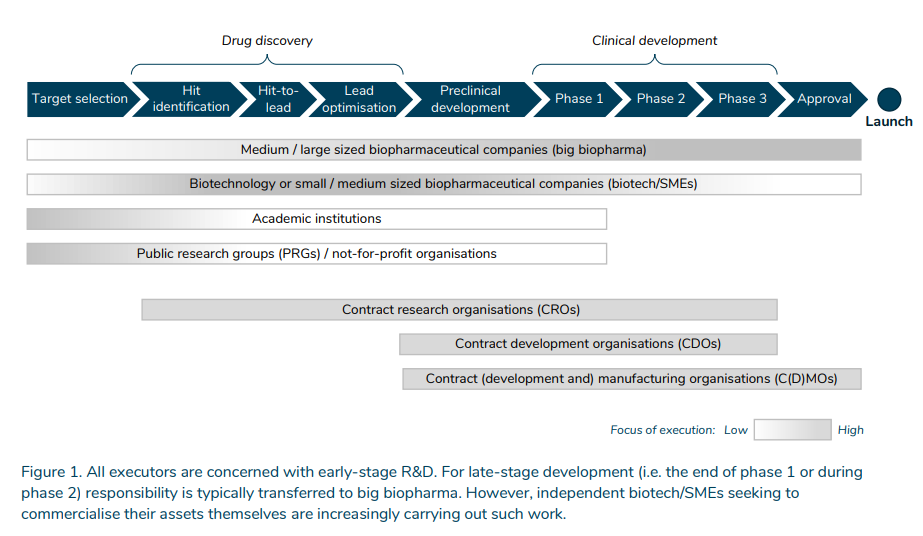
- Academic establishments and public analysis teams (PRGs) / not-for-profit organisations 1 are principally involved with goal choice, i.e. figuring out illness targets, though they might additionally play a job in later phases.
- Biotechnology firms (biotech) or small/medium-sized biopharmaceutical firms (SMEs) are most energetic in drug discovery, preclinical improvement and early-stage scientific improvement. Drug discovery entails discovering and optimising a drug candidate that interacts with the illness goal. In the preclinical improvement section, the protection and efficacy profiles of the drug candidate are examined in animal fashions and subsequently in human trials in clinical-development phases.
- Medium/large-sized biopharmaceutical firms (huge biopharma) are energetic all through the entire worth chain. They are the vital late-stage scientific improvement executors. The accountability is usually transferred to huge biopharma from the top of section 1 or throughout section 2. However, biotech/SMEs searching for to commercialise their property themselves are more and more finishing up such work.
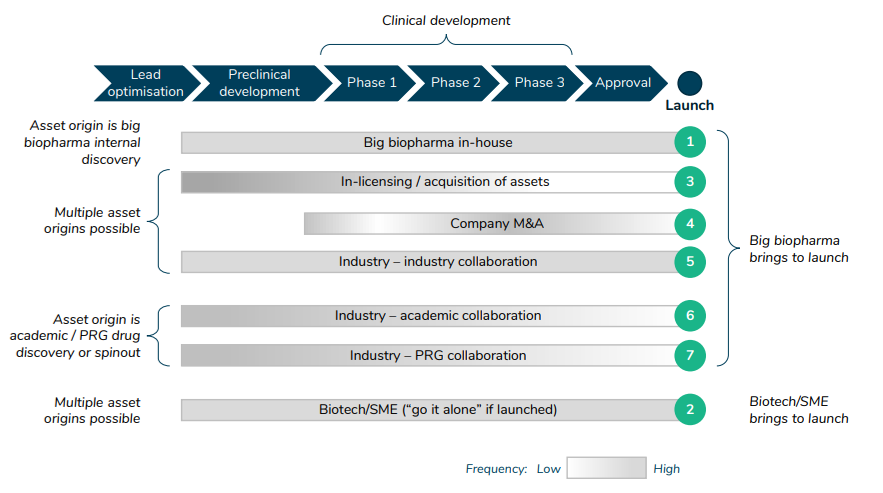
What are typical archetypes of how completely different stakeholders work together within the drug improvement course of?
The paper additionally lists 7 completely different archetypes for drug improvement.
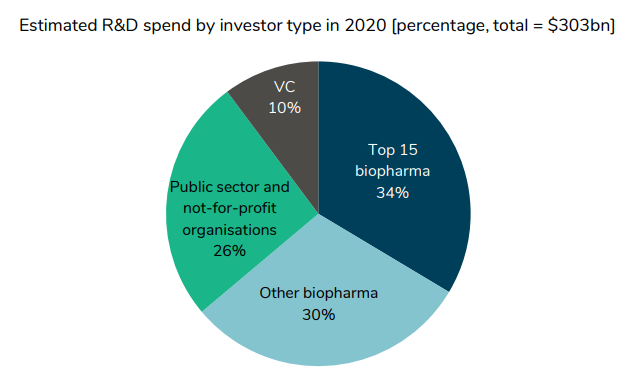
Who funds drug improvement?
About two-thirds comes from personal sector life sciences firms. However, we see that between 2011-2019, personal sector funding has grown. VC funding has grown by 14.2% per 12 months, biopharma by 4.1%, however public sector and not-for-profit solely grew by 1.1% and 0.8% respectively.
A case examine of how non-profits and biopharma funding work together is proven by the latest improvement of quite a few cystic fibrosis medicine.
Since only some therapies have been accessible to deal with the signs of cystic fibrosis (CF) within the late Nineteen Nineties, the Cystic Fibrosis Foundation (CFF) regarded to assist the event of disease-modifying therapies. CFF needed to make strategic investments in pharma firms aimed explicitly at cystic-fibrosis-therapy improvement. In 2000, CFF partnered with Aurora Biosciences to determine disease-modifying molecules. Vertex Pharma acquired Aurora Biosciences in 2001 however didn’t make investments closely on this CF franchise attributable to a heavy strategic deal with virology. When Kalydeco entered section 1 trials in 2006, CFF funded an extra $37m. The profitable outcomes of this section inspired Vertex to spend money on constructing extra R&D and commercialisation capabilities for the CF franchise. Moreover, CFF funded an extra $75m after section 2 trials started. After approval in 2012, Kalydeco grew to become commercially profitable. CFF benefited by promoting their royalty rights for Kalydeco in a $3.3bn deal they reinvested in CF analysis
How a lot does it price to develop a drug?
It is pricey:
Whether efficiently launched or not, an executing firm’s out-of-pocket R&D prices for one compound are an estimated $280–$380m. If together with the R&D prices of medicine that fail, nonetheless, the estimated out-of-pocket prices to the system for creating one permitted drug improve significantly to $1.2– $1.7bn (§2.3.2). Adding the price of capital, the whole R&D price to the system provides as much as an estimated $2.4–$3.2bn per single permitted drug
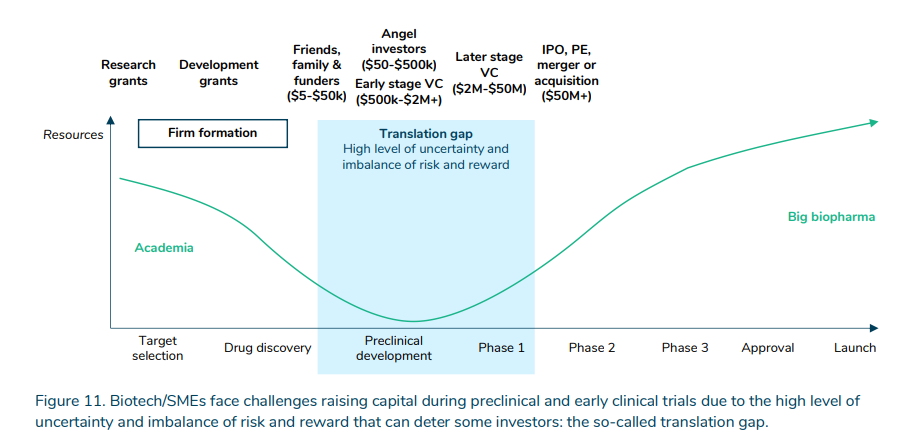
More than simply the expense, is the uncertainty. Government, non-profit and enterprise capital helps to bridge the “translation gap” between early stage fundamental scientific analysis into goal choice and drug discovery and into pre-clinical and scientific trials.
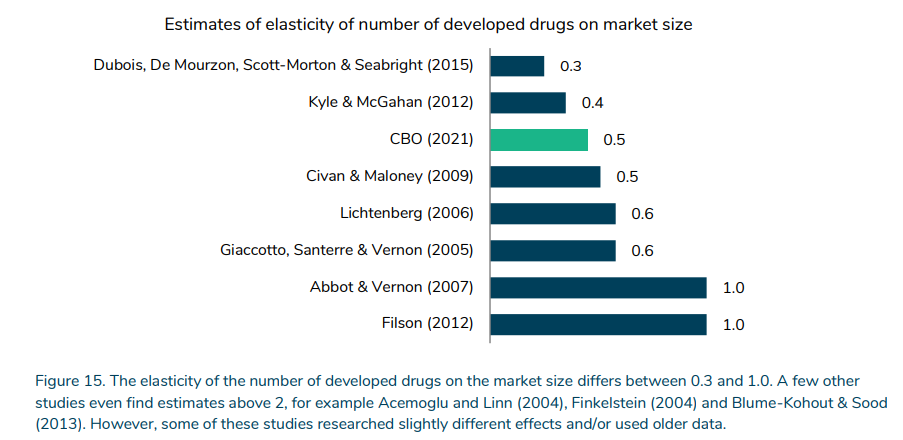
How responsive is R&D funding to drug revenues and/or market measurement?
A key determine is how responsive drug improvement is to anticipated income. This elasticity ranges from 0.3 to >2.0, however the determine under focuses on newer research the place the vary is 0.3 to 1.0.
The full report may be learn right here.
