[ad_1]
The yr 2022 was an excellent, however maybe not nice yr for innovation. According to the FDA’s Center for Drug Evaluation and Research’s (CDER) New Drug Therapy Approvals 2022 report, there have been 37 novel medication accredited in 2022. This quantity is down from the historic price of approvals between 2013-2021 (43.4 approvals per yr) and particularly down relative to the final 5 years (51.2 approvals per yr).

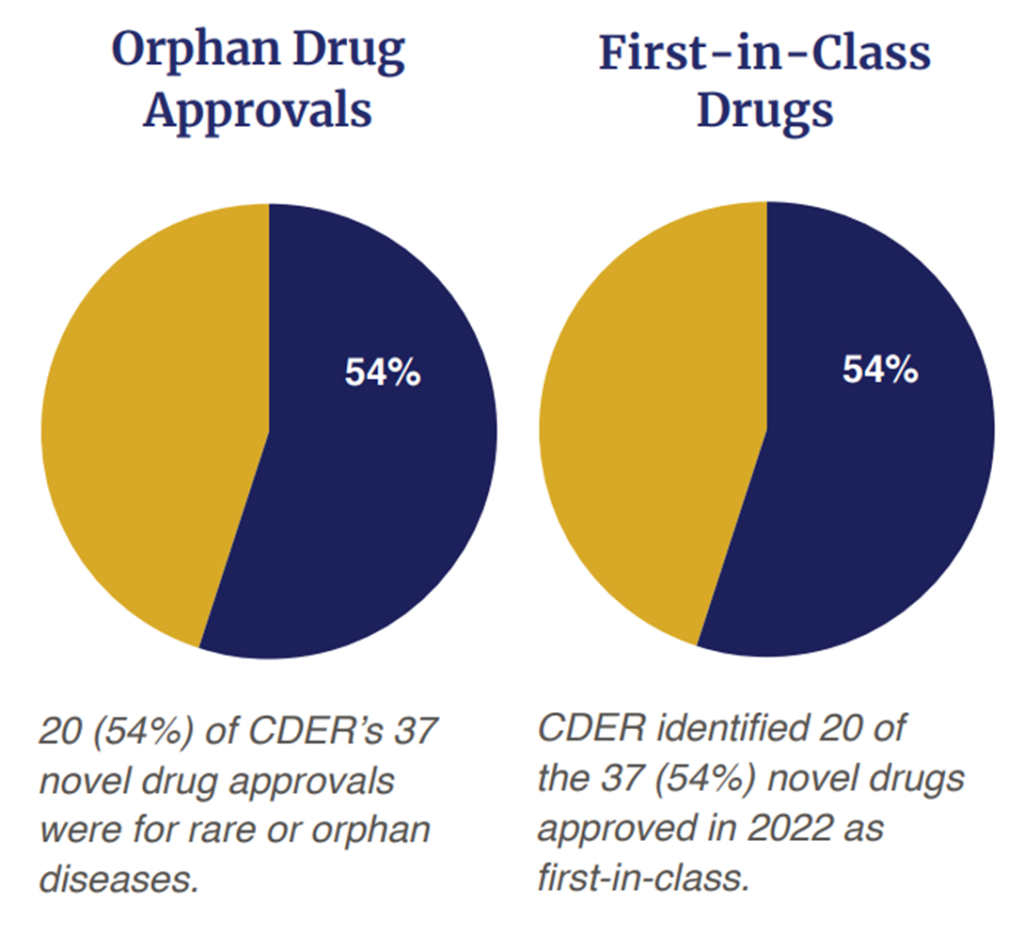
On the opposite hand, a majority of the medication (54%, 20 out of 37) had been first in school approvals. The period of precision medication can also be upon us as 54% (20 out of 37) had been for uncommon or orphan illnesses. Out of the 37 approvals, 32% (n=12) had been accredited by way of the Fast Track standing; 35% (n=13) had been accredited with a Breakthrough Therapy designation; 57% (n=21) had been accredited based mostly on a Priority Review Designation and 16% (n=6) had been accredited beneath the Accelerated Approval Program. In brief, 65% (n=24) of the 37 medication accredited obtained some sort of expedited evaluation.
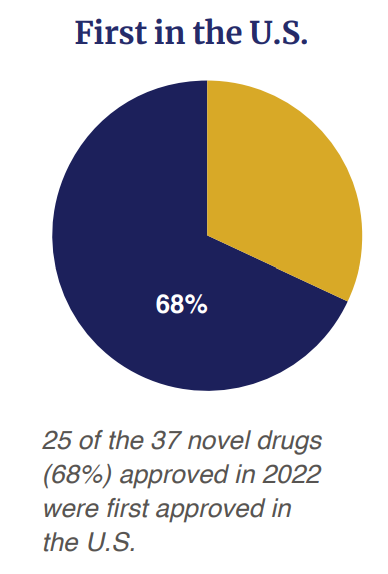
Of the 37 drug accredited, 68% (n=25) had been first accredited within the US earlier than some other nation.
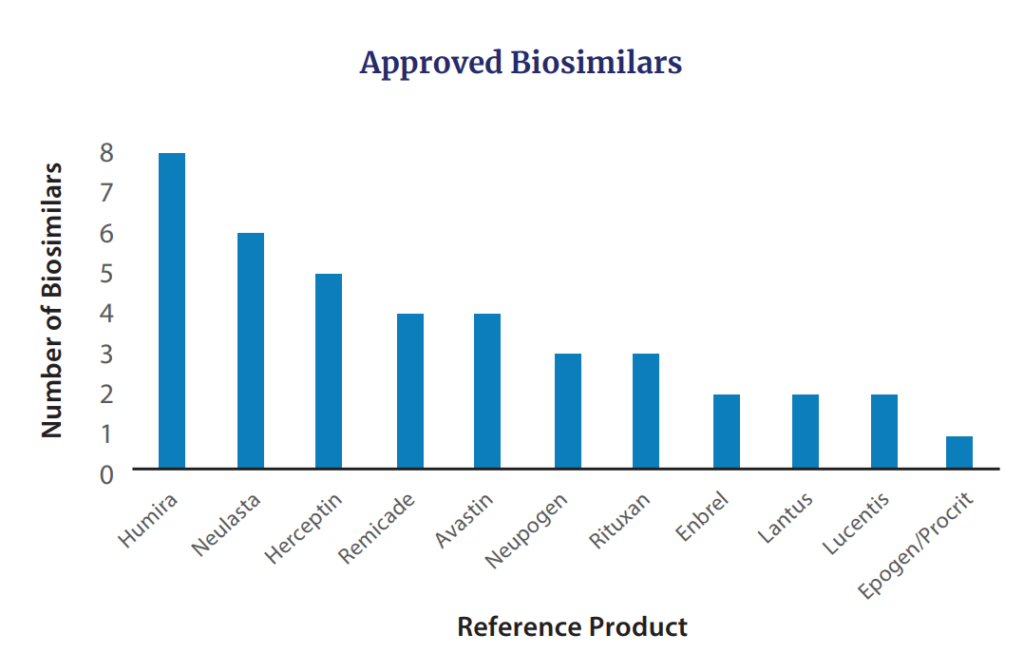
Additionally, in 2022 FDA accredited seven biosimilars for merchandise together with Avastin (n=2), Humira, Lucentis, Neulasta (n=2), and Neupogen. The cumuulative biosimilars accredited by FDA so far are within the determine beneath.
You can discover the total record of product approvals within the FDA report right here.
