[ad_1]
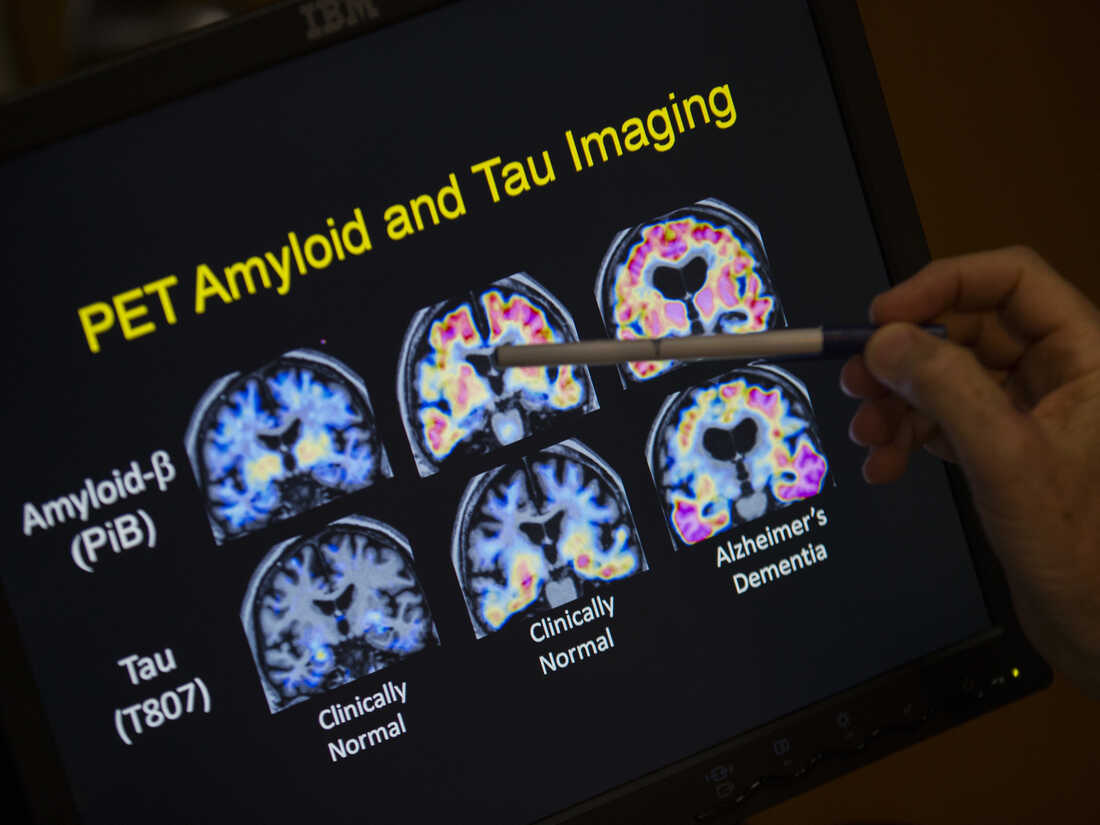
A health care provider factors to PET scan outcomes which can be a part of Alzheimer’s illness analysis. Much work within the area focuses a substance known as beta-amyloid. A brand new examine may take a look at whether or not that is the fitting goal.
Evan Vucci/AP
cover caption
toggle caption
Evan Vucci/AP
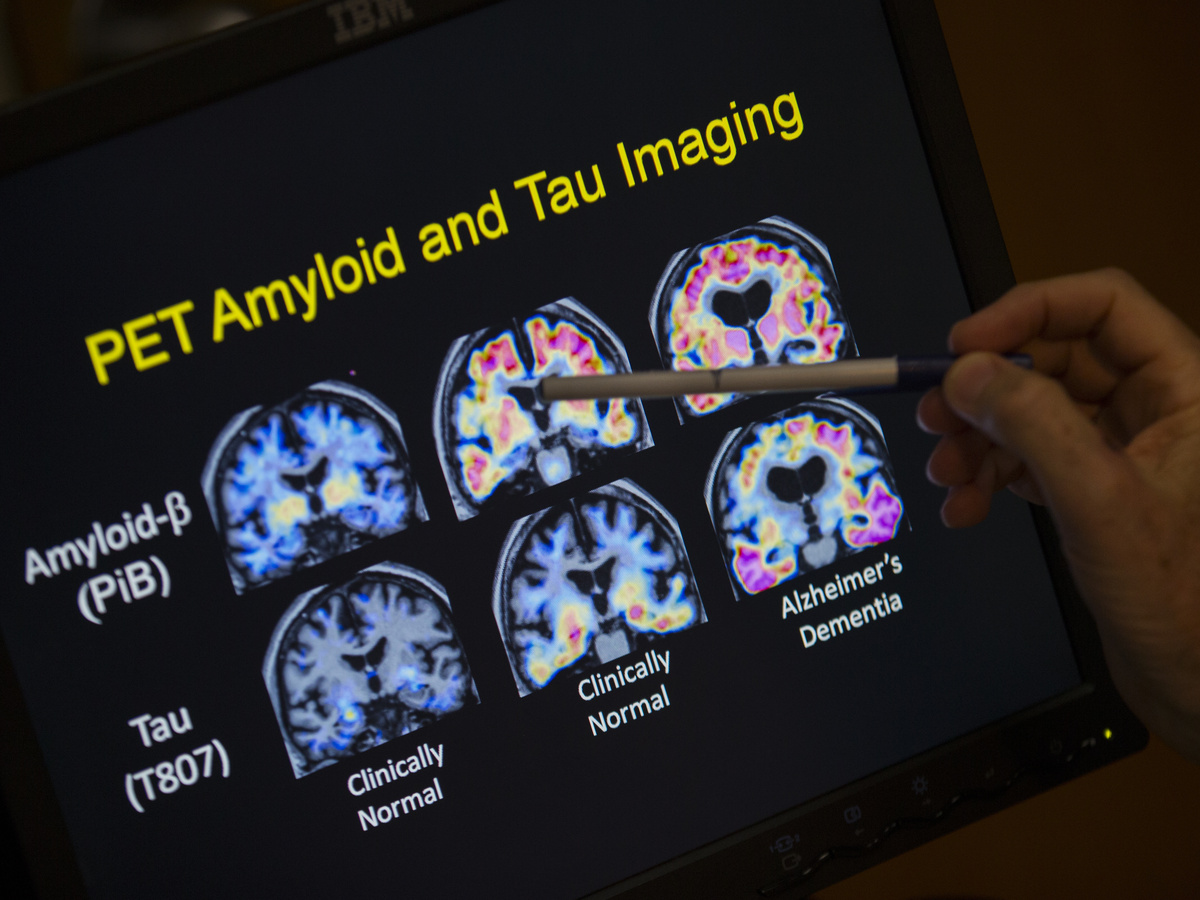
A health care provider factors to PET scan outcomes which can be a part of Alzheimer’s illness analysis. Much work within the area focuses a substance known as beta-amyloid. A brand new examine may take a look at whether or not that is the fitting goal.
Evan Vucci/AP
An concept that has propelled Alzheimer’s analysis for greater than 30 years is approaching its day of reckoning.
Scientists are launching a examine designed to make or break the speculation that Alzheimer’s is attributable to a sticky substance known as beta-amyloid. The examine will give an experimental anti-amyloid drug to folks as younger as 18 who’ve gene mutations that usually trigger Alzheimer’s to look of their 30s or 40s.
The examine comes after a number of experimental medicine have failed to stop declines in reminiscence and considering regardless that they succeeded in eradicating amyloid from the brains of sufferers within the early levels of Alzheimer’s. Those failures have eroded assist for the concept that amyloid is chargeable for a cascade of occasions that ultimately result in the demise of mind cells.
“Many of us consider that as the last word take a look at of the amyloid speculation,” says Dr. Randall Bateman, a professor of neurology at Washington University School of Medicine in St. Louis.”If that does not work, nothing will work.”
The new experiment, known as the DIAN-TU major Prevention Trial, is scheduled to start enrolling sufferers by the top of the 12 months.
An clarification with a historical past
The amyloid speculation might be traced to Dr. Alois Alzheimer, a pathologist who first described the illness that may bear his title in 1906.
Alzheimer was working at a psychiatric clinic in Munich, the place he had the prospect to conduct an post-mortem on a girl who died at 50 after experiencing reminiscence loss, disorientation, and hallucinations. He noticed that the lady’s mind had an “uncommon illness of the cerebral cortex,” together with “senile plaque” normally seen in a lot older folks.
In the Nineteen Eighties, scientists confirmed that these plaques had been fabricated from beta-amyloid, a substance that exists in lots of kinds within the mind, from single free-floating molecules to massive assemblies that kind the sticky plaques reported by Alzheimer.
Since that discovery, most efforts to deal with Alzheimer’s have concerned medicine that focus on numerous types of amyloid. And that method nonetheless is sensible, Bateman says.
“We have 30 years of strong information, hundreds of research that each one say that is adequate to trigger Alzheimer’s,” he says.
But doubts concerning the amyloid speculation have been rising because the record of drug failures has grown prior to now decade.
For instance, Bateman and a workforce of researchers had been unable to halt Alzheimer’s in a examine of sufferers who obtained the anti-amyloid drug gantenerumab.
“What we discovered was that it had reversed the amyloid plaques of their brains,” Bateman says. “We didn’t have proof of a thinking-memory profit.”
Even so, Bateman and lots of different scientists suppose it is too quickly to desert the amyloid speculation.
“Penicillin, a fantastic breakthrough, failed its first two medical trials,” Bateman says. “Fortunately, folks did not say, oh, the antibiotic idea is a foul thought and we must always quit on it.”
Hints of a profit
Bateman is inspired by outcomes from current research of anti-amyloid medicine, even those that haven’t prevented cognitive decline.
Gantenerumab, for instance, appeared to delay a number of mind modifications related to the demise of mind cells, he says.
And the experimental drug lecanemab did seem to decelerate the lack of reminiscence and considering in a examine of almost 1,800 folks with early Alzheimer’s illness, in response to a statement from the drug’s maker.
Many research of anti-amyloid medicine might have failed as a result of they got to individuals who already had amyloid plaques of their brains. At that time, Bateman says, it might not be doable to cease the method that finally kills off mind cells.
So Bateman is optimistic concerning the upcoming prevention trial, which is able to begin therapy a lot earlier.
“My prediction is it should work, and it’ll work fantastically,” he says. “If we are able to actually stop the plaques from beginning and taking off and people downstream modifications from going, my prediction is these folks won’t ever get Alzheimer’s.”
The prevention examine is predicated on the concept that when amyloid begins to construct up, it causes a sequence of modifications within the mind, says Dr. Eric McDade, a professor of neurology at Washington University who will oversee the experiment.
These modifications embrace the looks of poisonous tau tangles inside neurons, the lack of connections between neurons, irritation, and, finally, the demise of mind cells concerned in considering and reminiscence.
“What we’re attempting to do is to stop that amyloid pathology from growing within the first place,” McDade says.
That type of prevention, although, will imply beginning therapy lengthy earlier than signs seem.
“At the purpose of anyone having signs, we all know now that they in all probability have had amyloid of their mind for one to 20 years,” McDade says.
So the four-year examine will enroll about 160 folks from households with dominantly inherited Alzheimer’s illness. This type of dementia is attributable to uncommon, inherited gene mutations that trigger Alzheimer’s to develop in center age, usually in an individual’s 30s and 40s.
“The earliest they’ll are available is 25 years earlier than we anticipate they’d begin to develop signs,” McDade says. “For most of those households, that really places them of their mid 20s when we’ll begin this trial.”
Like the sooner examine that failed, this one will use the anti-amyloid drug gantenerumab.
The short-term aim is to ensure that amyloid plaques don’t seem. Then, researchers will look to see whether or not this prevents the looks of different markers of Alzheimer’s results on the mind.
One of those markers is the presence of neurofibrillary tangles, a poisonous model of a protein known as tau that kinds disorganized threads inside a neuron. These inside tangles disrupt a cell’s capacity to move chemical compounds and vitamins from place to position and to take care of connections with different cells.
Another marker is mind atrophy, a shrinkage in a number of mind areas attributable to the lack of neurons and the connections between them.
“If we stop amyloid pathology from growing and these different markers proceed to develop and unfold,” McDade says, “this might be among the finest methods to say, pay attention, amyloid is de facto not what we must be focusing on.”
[ad_2]



