[ad_1]
The Digital Therapeutics Alliance defines a digital therapeutic (DTx) as “evidence-based therapeutic interventions that are driven by high-quality software programs to prevent, manage, or treat a medical disorder or disease.” One key query is what components do US payers take note of when evaluating DTx and the way does that differ from normal prescription drugs. A paper by Gomez Lumbreras et al. (2024) held digital focus teams with 21 US payers to seek out the reply. Key issues embrace:
- Need for Evidence. Almost all survey respondents (n = 19/21 90%) indicated they might require a medical trial to think about protection of the product. This proof contains knowledge on efficacy, effectiveness and worth (together with cost-effectiveness perspective)
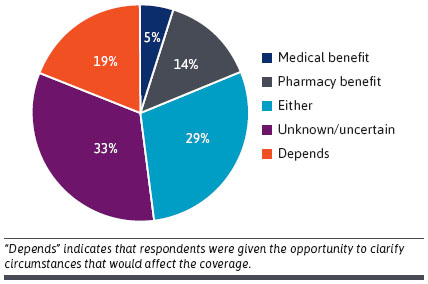
- DTx Coverage: Medical, Pharmacy or Other? Many respondents had been unsure if reimbursement ought to undergo medical or pharmacy advantages. The majority thought it could in all probability be the pharmacy and therapeutics committee (n = 15/21 71%), however , a number of contributors answered “other” (n = 6/21, 29%) [see Figure below]
- FDA Regulation and Pending Legislation. Overall, 14/21 (66.7%) respondents would require an FDA analysis of the DTx product for it to be thought-about for protection (particularly if coated beneath the pharmacy profit). Other respondents indicated that FDA analysis was helpful however not all the time required for protection. Several payers cited the necessity for proof past the necessities of the FDA to think about a DTx product for protection (e.g., effeciveness, worth).
- Reimbursement: NDC vs. CPT. A prescription could be mandatory for a lot of well being plans to reimburse a DTx product provided that many insurance policies exclude reimbursement for over-the-counter merchandise. Participants broadly agreed {that a} coding system could be required, and {that a} Current Procedural Terminology (CPT) code or National Drug Code (NDC) could be probably the most environment friendly methods to make sure reimbursement.
- Barriers. Barriers talked about embrace sturdiness of therapy impact, price of merchandise, and mechanisms for reimbursement/fee. Other points included the position of DTx merchandise on affected person engagement and therapy adherence. Many perceived that DTx weren’t “bona fide” therapies partially as a result of some thought-about them simply “apps” and comparable variations could possibly be downloaded on-line totally free.
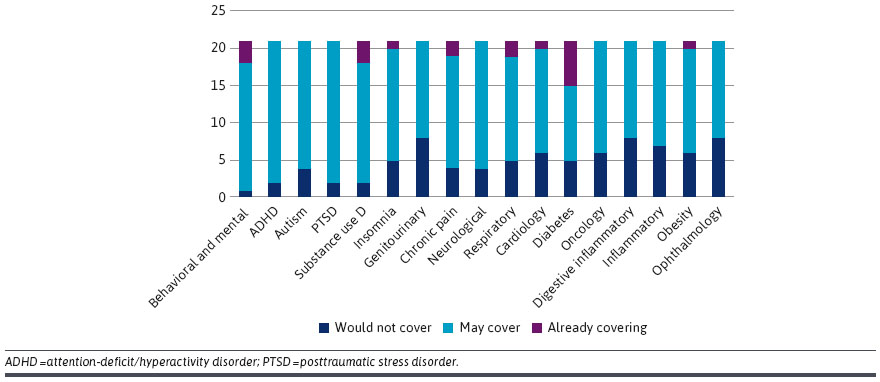
- Payer Management. Some claimed that utilization administration insurance policies (e..g, prior authorization, step edits, amount limits) could possibly be used for DTx simply as they’re for pharmaceuticals. Others steered {that a} DTx product could possibly be a part of a care administration program somewhat than protecting it individually. A couple of contributors defined that their organizations had been at the moment protecting DTx merchandise as a part of medical packages.
You can learn the complete article with useful quotations right here.
