[ad_1]
When a affected person is identified with most cancers, one of the vital necessary steps is examination of the tumor beneath a microscope by pathologists to find out the most cancers stage and to characterize the tumor. This info is central to understanding medical prognosis (i.e., probably affected person outcomes) and for figuring out probably the most acceptable remedy, comparable to present process surgical procedure alone versus surgical procedure plus chemotherapy. Developing machine studying (ML) instruments in pathology to help with the microscopic evaluate represents a compelling analysis space with many potential functions.
Previous research have proven that ML can precisely determine and classify tumors in pathology photos and may even predict affected person prognosis utilizing identified pathology options, such because the diploma to which gland appearances deviate from regular. While these efforts concentrate on utilizing ML to detect or quantify identified options, various approaches provide the potential to determine novel options. The discovery of latest options might in flip additional enhance most cancers prognostication and remedy choices for sufferers by extracting info that isn’t but thought-about in present workflows.
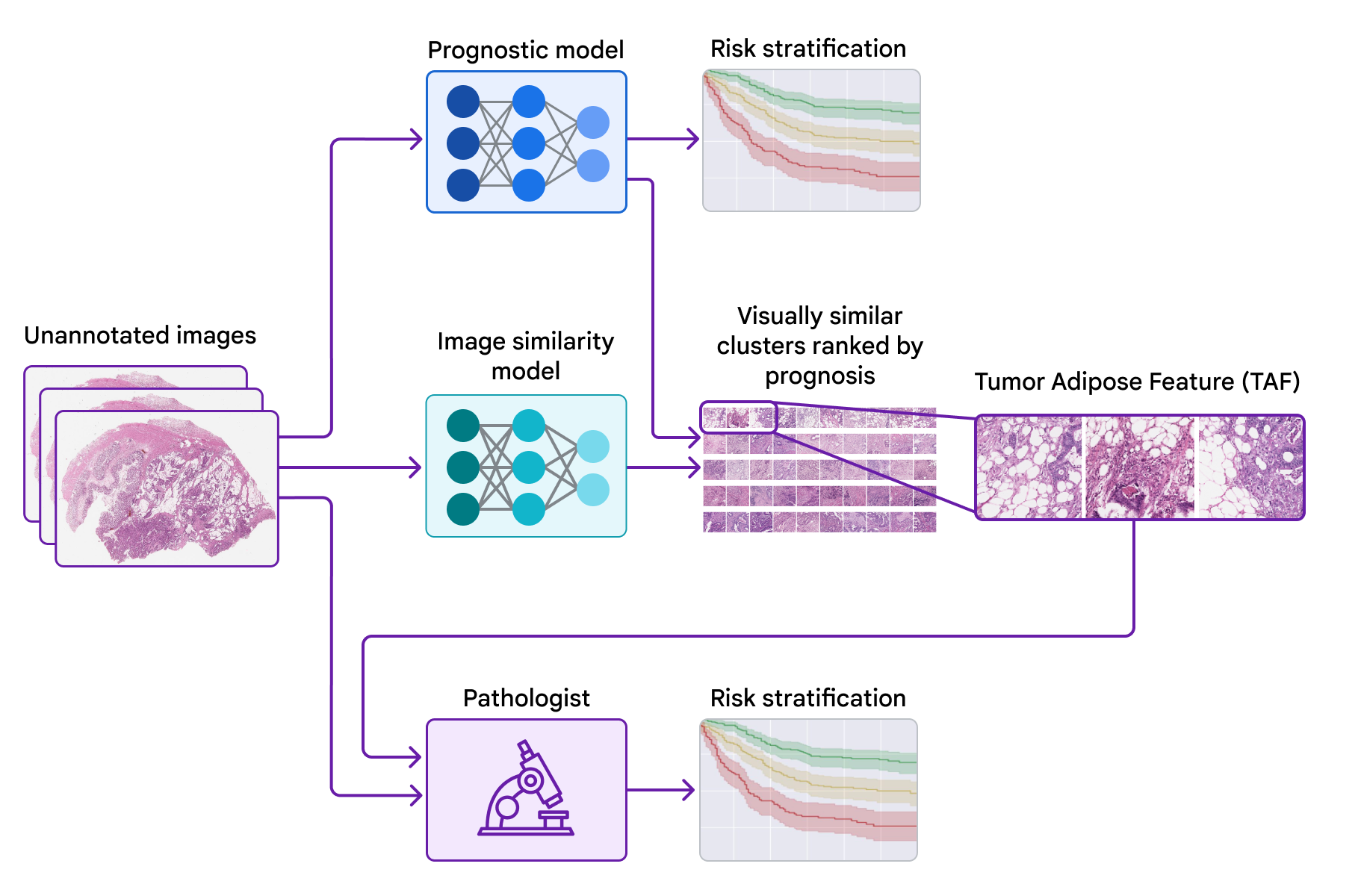
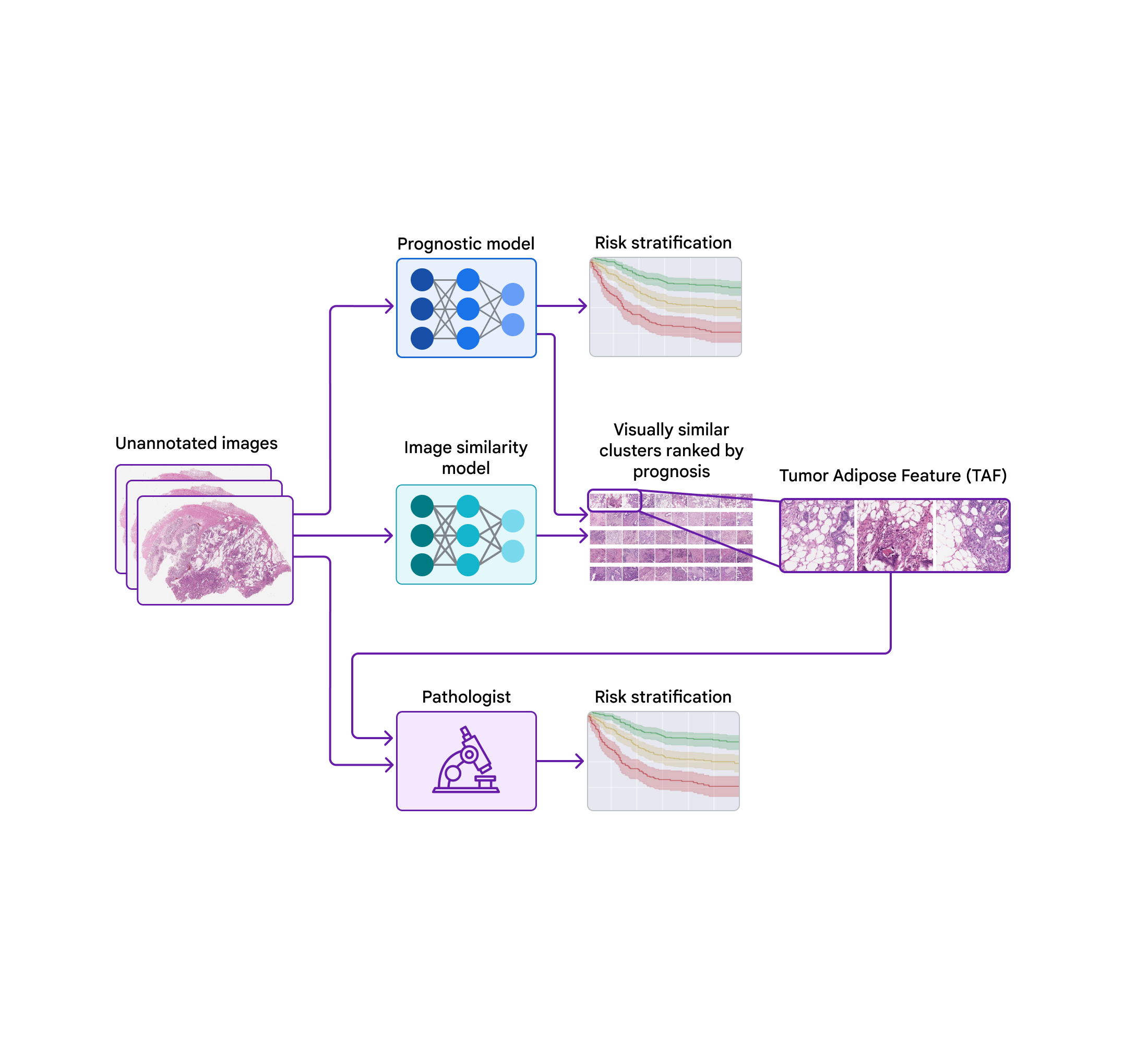
Today, we’d prefer to share progress we’ve revamped the previous few years in the direction of figuring out novel options for colorectal most cancers in collaboration with groups on the Medical University of Graz in Austria and the University of Milano-Bicocca (UNIMIB) in Italy. Below, we are going to cowl a number of levels of the work: (1) coaching a mannequin to foretell prognosis from pathology photos with out specifying the options to make use of, in order that it could possibly study what options are necessary; (2) probing that prognostic mannequin utilizing explainability methods; and (3) figuring out a novel characteristic and validating its affiliation with affected person prognosis. We describe this characteristic and consider its use by pathologists in our just lately revealed paper, “Pathologist validation of a machine-learned feature for colon cancer risk stratification”. To our information, that is the primary demonstration that medical consultants can study new prognostic options from machine studying, a promising begin for the way forward for this “learning from deep learning” paradigm.
Training a prognostic mannequin to study what options are necessary
One potential strategy to figuring out novel options is to coach ML fashions to immediately predict affected person outcomes utilizing solely the photographs and the paired final result information. This is in distinction to coaching fashions to foretell “intermediate” human-annotated labels for identified pathologic options after which utilizing these options to foretell outcomes.
Initial work by our crew confirmed the feasibility of coaching fashions to immediately predict prognosis for a wide range of most cancers sorts utilizing the publicly obtainable TCGA dataset. It was particularly thrilling to see that for some most cancers sorts, the mannequin’s predictions had been prognostic after controlling for obtainable pathologic and medical options. Together with collaborators from the Medical University of Graz and the Biobank Graz, we subsequently prolonged this work utilizing a big de-identified colorectal most cancers cohort. Interpreting these mannequin predictions grew to become an intriguing subsequent step, however widespread interpretability methods had been difficult to use on this context and didn’t present clear insights.
Interpreting the model-learned options
To probe the options utilized by the prognostic mannequin, we used a second mannequin (skilled to determine picture similarity) to cluster cropped patches of the big pathology photos. We then used the prognostic mannequin to compute the typical ML-predicted threat rating for every cluster.
One cluster stood out for its excessive common threat rating (related to poor prognosis) and its distinct visible look. Pathologists described the photographs as involving excessive grade tumor (i.e., least-resembling regular tissue) in shut proximity to adipose (fats) tissue, main us to dub this cluster the “tumor adipose feature” (TAF); see subsequent determine for detailed examples of this characteristic. Further evaluation confirmed that the relative amount of TAF was itself extremely and independently prognostic.
| Left: H&E pathology slide with an overlaid heatmap indicating areas of the tumor adipose characteristic (TAF). Regions highlighted in crimson/orange are thought-about to be extra probably TAF by the picture similarity mannequin, in comparison with areas highlighted in inexperienced/blue or areas not highlighted in any respect. Right: Representative assortment of TAF patches throughout a number of circumstances. |
Validating that the model-learned characteristic can be utilized by pathologists
These research offered a compelling instance of the potential for ML fashions to foretell affected person outcomes and a methodological strategy for acquiring insights into mannequin predictions. However, there remained the intriguing questions of whether or not pathologists might study and rating the characteristic recognized by the mannequin whereas sustaining demonstrable prognostic worth.
In our most up-to-date paper, we collaborated with pathologists from the UNIMIB to research these questions. Using instance photos of TAF from the earlier publication to study and perceive this characteristic of curiosity, UNIMIB pathologists developed scoring pointers for TAF. If TAF was not seen, the case was scored as “absent”, and if TAF was noticed, then “unifocal”, “multifocal”, and “widespread” classes had been used to point the relative amount. Our examine confirmed that pathologists might reproducibly determine the ML-derived TAF and that their scoring for TAF offered statistically important prognostic worth on an unbiased retrospective dataset. To our information, that is the primary demonstration of pathologists studying to determine and rating a particular pathology characteristic initially recognized by an ML-based strategy.
Putting issues in context: studying from deep studying as a paradigm
Our work is an instance of individuals “learning from deep learning”. In conventional ML, fashions study from hand-engineered options knowledgeable by current area information. More just lately, within the deep studying period, a mix of large-scale mannequin architectures, compute, and datasets has enabled studying immediately from uncooked information, however that is usually on the expense of human interpretability. Our work {couples} the usage of deep studying to foretell affected person outcomes with interpretability strategies, to extract new information that might be utilized by pathologists. We see this course of as a pure subsequent step within the evolution of making use of ML to issues in drugs and science, shifting from the usage of ML to distill current human information to folks utilizing ML as a software for information discovery.
Acknowledgements
This work wouldn’t have been doable with out the efforts of coauthors Vincenzo L’Imperio, Markus Plass, Heimo Muller, Nicolò’ Tamini, Luca Gianotti, Nicola Zucchini, Robert Reihs, Greg S. Corrado, Dale R. Webster, Lily H. Peng, Po-Hsuan Cameron Chen, Marialuisa Lavitrano, David F. Steiner, Kurt Zatloukal, Fabio Pagni. We additionally admire the assist from Verily Life Sciences and the Google Health Pathology groups – specifically Timo Kohlberger, Yunnan Cai, Hongwu Wang, Kunal Nagpal, Craig Mermel, Trissia Brown, Isabelle Flament-Auvigne, and Angela Lin. We additionally admire manuscript suggestions from Akinori Mitani, Rory Sayres, and Michael Howell, and illustration assist from Abi Jones. This work would additionally not have been doable with out the assist of Christian Guelly, Andreas Holzinger, Robert Reihs, Farah Nader, the Biobank Graz, the efforts of the slide digitization crew on the Medical University Graz, the participation of the pathologists who reviewed and annotated circumstances throughout mannequin growth, and the technicians of the UNIMIB crew.