[ad_1]
A federal choose in Texas issued a ruling Friday to revoke the Food and Drug Administration’s practically 23-year-old approval of the secure and efficient abortion and miscarriage medicine, mifepristone. Although anticipated, the ruling throws into query the FDA’s authority over all medicines and threatens to weaken the nation’s premier drug improvement pipeline, business leaders and authorized consultants say.
In a public letter that circulated over the weekend, executives and leaders of the biotechnology and pharmaceutical industries condemned the ruling and known as for its reversal together with “acceptable restitution” of the FDA’s authority.
As of Monday afternoon, the letter had round 400 signatures and was accumulating extra. Among them are huge gamers within the business, together with Pfizer CEO Albert Bourla; Alisha Alaimo, president of Biogen; Christopher Tan, an govt for Merck & Co.; Imran Nasrullah, a vp for Bayer Pharmaceuticals; and a senior medical chief at Novartis, Nancy Lewis. But the overwhelming majority are from smaller biotech corporations, who stand to lose probably the most from downstream results of the ruling, issued by District Judge Matthew Kacsmaryk.
In the letter, the biopharma leaders chastised Kacsmaryk as an activist choose, emphasizing that he has “no scientific coaching,” and that his ruling “ignores many years of scientific proof,” discovering that mifepristone is “safer than Tylenol, practically all antibiotics and insulin.” The ruling additionally “basically undermined” the FDA’s authority to approve and regulate secure, efficient medicines for Americans, which “creates uncertainty for your complete biopharma business.”
Fewer medication, much less innovation
That uncertainty, they are saying, will chill investments in drug improvement and endanger innovation. The business is already “inherently dangerous,” they observe, referencing that almost all drug merchandise do not efficiently make it via medical trials, regardless of requiring tens of millions of {dollars} of funding over years, and infrequently many years, of improvement. With the few medication that do make it to market, drug makers depend on having years of revenue from gross sales that may assist help riskier analysis and improvement packages.
The US biopharma business is singular in its sheer dimension, productiveness, and innovation. It produces probably the most FDA-approved medication of any nation, and has a number of the highest proportions of medication with novel constructions and mechanisms. The US can also be an outlier in producing extra medication for unmet medical wants than others and leans on biotech corporations and tutorial establishments, slightly than simply pharmaceutical corporations, greater than others.
But that preeminence depends on a dependable regulatory course of, which is now in query with final week’s ruling on mifepristone’s. “Judicial activism won’t cease right here,” the biopharma leaders wrote. “If courts can overturn drug approvals with out regard for science or proof, or for the complexity required to completely vet the security and efficacy of recent medication, any medication is in danger for a similar consequence as mifepristone,” they stated.
The leaders acknowledge that FDA processes and oversight are “not good,” however have “resulted in many years of unsurpassed medical innovation.”
In a press release Friday, President Joe Biden echoed their considerations concerning the ruling’s broader implications. “If this ruling had been to face, then there might be nearly no prescription, authorized by the FDA, that might be secure from these sorts of political, ideological assaults,” the president stated.
Legal consultants have stated the identical. “If your approval will be withdrawn at a second’s discover by a single choose, it’s actually form of a scary factor,” I. Glenn Cohen, a Harvard Law School professor and bioethics professional, instructed The New York Times.
Safe, efficient, however not the one choice
The Department of Justice, representing the FDA, is interesting the ruling. The FDA launched a press release Friday, saying: “FDA stands behind its dedication that mifepristone is secure and efficient below its authorized situations of use for medical termination of early being pregnant, and believes sufferers ought to have entry to FDA-approved medicines that FDA has decided to be secure and efficient for his or her supposed makes use of.”
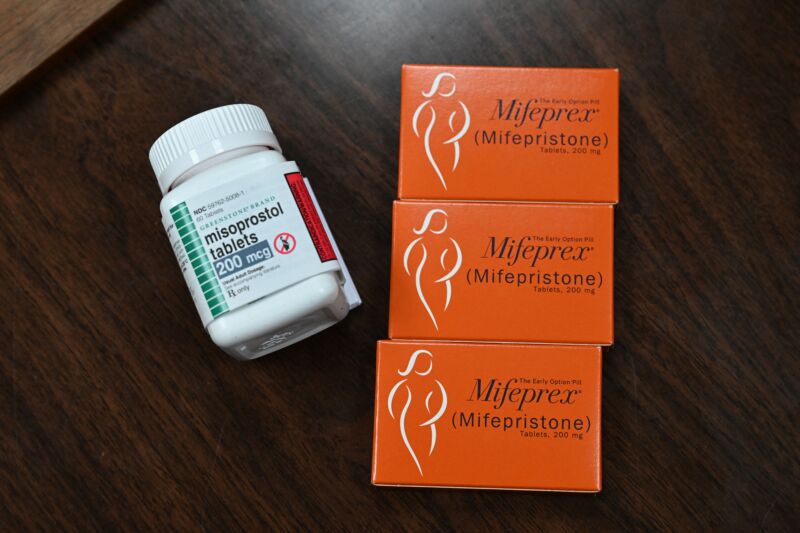
For now, mifepristone stays accessible. It is utilized in mixture with one other drug known as misoprostol to terminate an early being pregnant. The FDA has authorized its use within the first 10 weeks (70 days) of a being pregnant. (Pregnancy is dated from the primary day of the final menstrual interval, not an estimated day of conception, which is roughly two weeks after the primary day of the final menstrual interval, or when.) The World Health Organization, nonetheless, says the routine is secure as much as 12 weeks (84 days) of a being pregnant.
Mifepristone is an artificial steroid that blocks progesterone, which performs an important function in sustaining a being pregnant by controlling the atmosphere of the uterus. Misoprostol is an artificial prostaglandin (a hormone-like substance) that induces contractions and cervical dilation. The results of the routine is cramping, bleeding, and termination of the being pregnant. Medication abortion is utilized in over half of the abortions within the US.
If mifepristone turns into unavailable within the US, clinicians are ready to make use of a misoprostol-only protocol, which is often used outdoors the US. It is secure, efficient, and works extra rapidly, but it surely tends to contain extra uncomfortable side effects, resembling nausea, diarrhea, and vomiting. But with Roe v. Wade overturned final June, reproductive well being care stays below fixed, intense menace within the US. As of now, 12 states are imposing whole abortion bans, and lots of others have a spread of restrictions.
