[ad_1]
Pham et al. (2023) makes use of information on regulatory selections and well being expertise assessments (HTAs) in Australia, Canada, and the UK and compares them to the medication which might be FDA-approved within the US. They discover that:
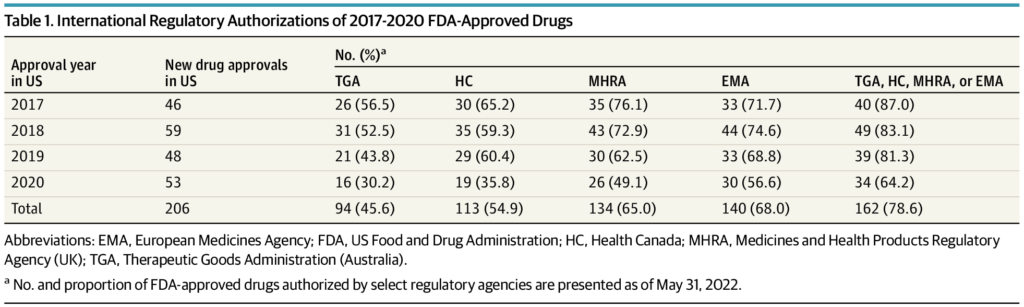
The FDA authorized 206 new medication in 2017 by 2020, of which 162 (78.6%) have been granted advertising and marketing authorization by no less than 1 different regulatory company at a median (IQR) delay of 12.1 (17.7) months following US approval. Conversely, 5 FDA-approved medication have been refused advertising and marketing authorization by a global regulatory company because of unfavorable benefit-to-risk assessments. An extra 42 FDA-approved medication acquired detrimental reimbursement suggestions from HTA companies in Australia, Canada, or the UK because of uncertainty of scientific advantages or unacceptably excessive costs. The median (IQR) US price of the 47 medication refused authorization or not beneficial for reimbursement by a global company was $115 281 ($166 690) per affected person per yr. Twenty medication have been for oncology indications, and 36 have been authorized by the FDA by expedited regulatory pathways or the Orphan Drug Act.
The full article is right here.
