[ad_1]
This story shall be up to date as extra info turns into accessible.
An extensively drug-resistant bacterial pressure is spreading within the US for the primary time and inflicting an alarming outbreak linked to synthetic tears eye drops, based on an alert launched Wednesday night from the Centers for Disease Control and Prevention. So far, the germ has triggered numerous infections in 55 folks in 12 states, killing one and leaving others hospitalized and with everlasting imaginative and prescient loss.
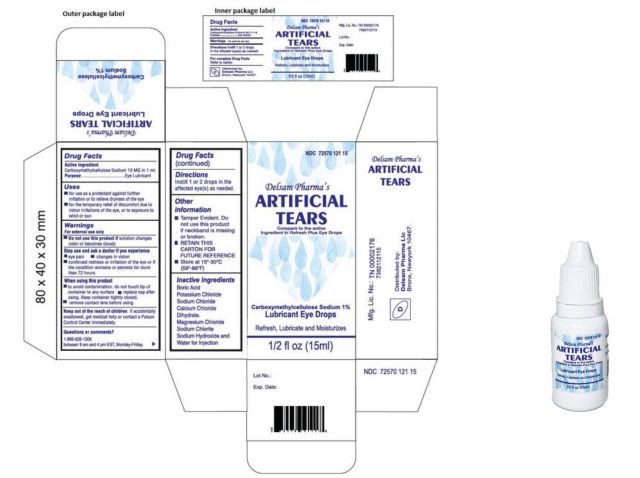
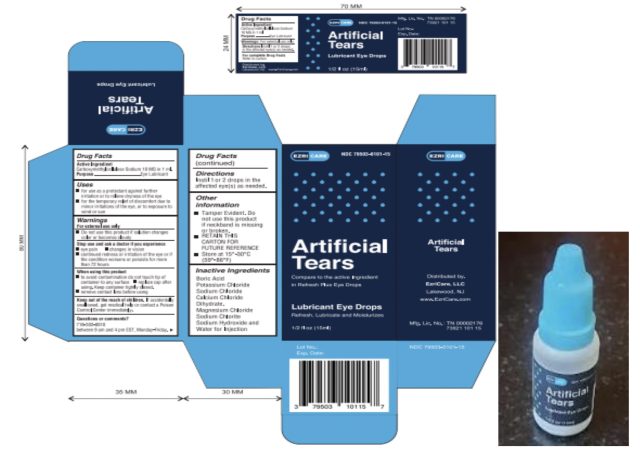
Infected sufferers reported utilizing greater than 10 manufacturers of synthetic tears collectively, with some sufferers utilizing a number of manufacturers. But the commonest model used among the many sufferers was EzriCare Artificial Tears, a preservative-free product offered by Walmart, Amazon, and different retailers.
No remembers have been introduced by the Food and Drug Administration, however the CDC recommends clinicians and sufferers cease utilizing EzriCare Artificial Tears merchandise pending further steerage from CDC and the FDA. The producer of EzriCare Artificial Tears introduced that it plans to recall the product, which can also be offered as Delsam Pharma’s Artificial Tears.
Formidable foe
The perpetrator behind the outbreak is a pressure of Pseudomonas aeruginosa, a particularly versatile, innately drug-resistant bacterium that lurks within the setting, significantly freshwater. It is thought to trigger numerous pores and skin, wound, burn, lung, and systemic infections. It most frequently strikes folks in immune-compromised states, resembling these with cystic fibrosis, and has a repute for sparking outbreaks in well being care settings, significantly amongst folks with indwelling units, like catheters and respiratory tubes. In hospital settings, it lurks in sinks, icemakers, machine washers, respiratory remedy gear, and on cleaning soap bars.
In the present outbreak, 35 of the 55 contaminated sufferers have been linked to 4 clusters of circumstances in well being care amenities. Among these 4 healthcare-associated clusters, the EzriCare Artificial Tears product was the one widespread product among the many amenities. CDC investigators additionally discovered the outbreak P. aeruginosa pressure in opened containers of EzriCare Artificial Tears bottles, which have been manufactured in numerous heaps and picked up from sufferers in two completely different states.
The outbreak pressure is a uncommon, extensively drug-resistant pressure with a mouthful of a reputation: Verona Integron-mediated Metallo-β-lactamase (VIM) and Guiana-Extended Spectrum-β-Lactamase (GES)-producing carbapenem-resistant P. aeruginosa—or VIM-GES-CRPA for brief.
While multi-drug resistant P. aeruginosa strains have lengthy posed a menace within the US and elsewhere, that is the primary time VIM-GES-CRPA has been discovered spreading within the US. The pressure is resistant to numerous antibiotic weapons, together with: cefepime, ceftazidime, piperacillin-tazobactam, aztreonam, carbapenems, ceftazidime-avibactam, ceftolozane-tazobactam, fluoroquinolones, polymyxins, amikacin, gentamicin, and tobramycin, the CDC reported.
So far, antibiotic susceptibility testing on three outbreak isolates means that the VIM-GES-CRPA pressure continues to be inclined to cefiderocol, a more recent antibiotic that acquired FDA approval in 2019 to deal with multidrug-resistant urinary tract infections.
In the present outbreak, which started in May 2022, investigators have remoted the outbreak pressure from 13 sputum or bronchial washes, 11 cornea swabs, seven urine samples, two blood samples, 25 rectal swabs, and 4 different nonsterile sources. The sufferers introduced in inpatient and outpatient settings with a variety of infections. Those embody eye infections—an infection of the cornea (keratitis) and an infection of tissue or fluids contained in the eyeball (endophthalmitis)— to respiratory infections, urinary tract infections, and sepsis. The affected person who died had a systemic an infection.
The circumstances to date occurred in 12 states: California, Colorado, Connecticut, Florida, New Jersey, New Mexico, New York, Nevada, Texas, Utah, Washington, and Wisconsin.
Response
In a assertion on February 1, EzriCare, LLC stated that’s cooperating with the CDC and FDA on the investigation. “As of right this moment, we aren’t conscious of any testing that definitively hyperlinks the Pseudomonas aeruginosa outbreak to EzriCare Artificial Tears,” the corporate stated. “Nonetheless, we instantly took motion to cease any additional distribution or sale of EzriCare Artificial Tears. To the best extent potential, we have now been contacting clients to advise them in opposition to continued use of the product.”
EzriCare famous that it solely has restricted involvement with the unreal tears product—which can also be marketed below different manufacturers, the corporate famous, with out figuring out another manufacturers. “EzriCare, LLC’s solely position in introducing the product to the market was to design an exterior label and to promote it to our clients,” the corporate stated. The eye drops are manufactured in India by Global Pharma Healthcare PVT Limited and imported into the United States by Aru Pharma Inc.
Global Pharma Healthcare posted a press launch on its web site dated February 1 saying that it’s voluntarily recalling its synthetic tears merchandise, although no formal recall discover has been posted by the FDA.