[ad_1]
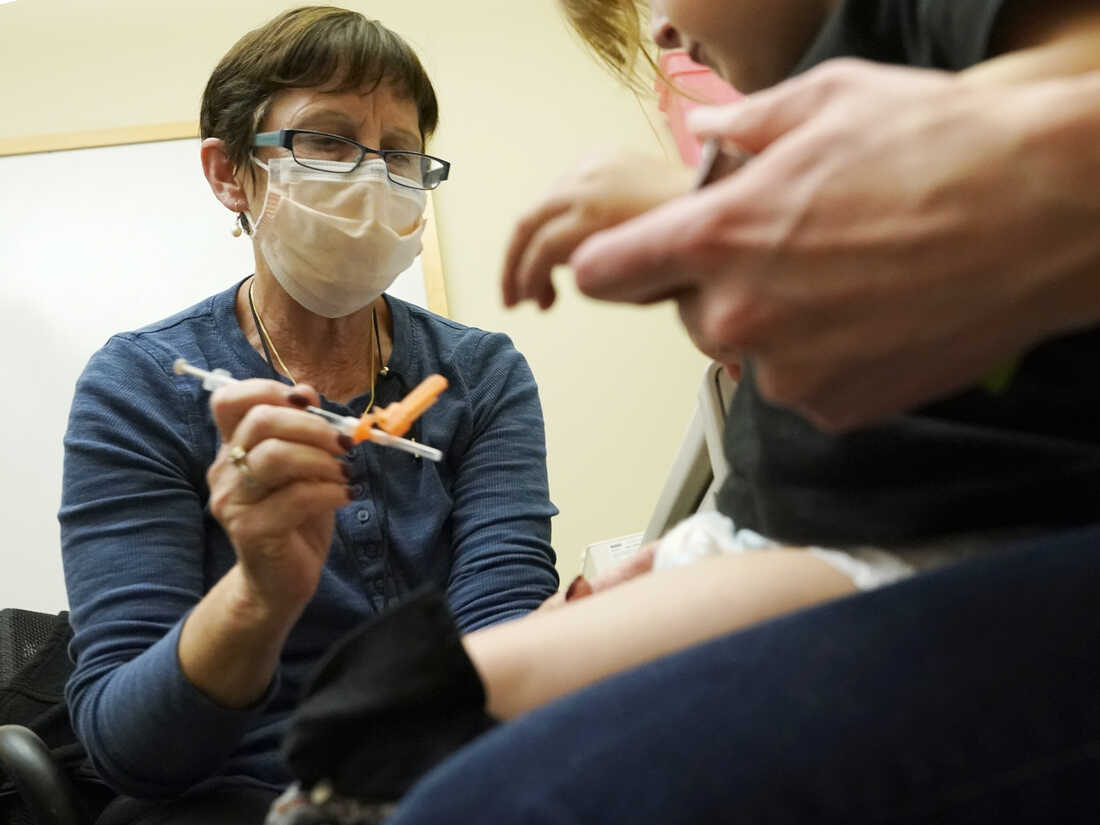
A nurse at a University of Washington Medical Center clinic in Seattle provides a Pfizer COVID-19 vaccine shot to a 20-month-old youngster on June 21.
Ted S. Warren/AP
conceal caption
toggle caption
Ted S. Warren/AP
A nurse at a University of Washington Medical Center clinic in Seattle provides a Pfizer COVID-19 vaccine shot to a 20-month-old youngster on June 21.
Ted S. Warren/AP
U.S. regulators on Thursday cleared doses of the up to date COVID-19 vaccines for kids youthful than age 5.
The Food and Drug Administration’s resolution goals to raised defend the littlest youngsters amid an uptick in COVID-19 circumstances across the nation — at a time when youngsters’s hospitals already are full of tots affected by different respiratory sicknesses together with the flu.
“Vaccination is one of the simplest ways we all know to assist forestall the intense outcomes of COVID-19, equivalent to hospitalization and loss of life,” Dr. Peter Marks, FDA’s vaccine chief, advised The Associated Press.
Omicron-targeted booster pictures made by Moderna and rival Pfizer already had been open to everybody 5 and older.
The FDA now has licensed use of the tweaked pictures beginning at age 6 months — however simply who’s eligible relies on what number of vaccinations they’ve already had, and which type. Only about 5% of kids beneath age 5 have gotten the total major collection since vaccinations for the littlest youngsters started in June.
The FDA determined that:
–Children beneath age 6 who’ve already gotten two unique doses of Moderna’s COVID-19 vaccine can get a single booster of Moderna’s up to date formulation if it has been no less than two months since their final shot.
–Pfizer’s vaccine requires three preliminary doses for tots beneath age 5 — and those that have not completed that vaccination collection will get the unique formulation for the primary two pictures and the omicron-targeted model for his or her third shot.
The Centers for Disease Control and Prevention is anticipated to log off quickly, the ultimate step for pictures to start.
Marks mentioned the bivalent vaccine is protected for tots and can assist mother and father “hold the safety for these youngsters as updated as potential.”
But youngsters beneath 5 who already received all three Pfizer doses aren’t but eligible for an up to date booster.
For now, “the excellent news is they’re in all probability fairly well-protected,” Marks mentioned.
The FDA expects information from Pfizer and its companion BioNTech someday subsequent month to find out whether or not these tots will want an omicron-targeted booster “and we’ll act on that as quickly as we will,” he mentioned.
For mother and father who have not but gotten their youngsters vaccinated, it isn’t too late — particularly as “we’re coming into a part when COVID-19 circumstances are rising,” Marks mentioned.
The up to date vaccines from Moderna and Pfizer are mixture pictures, containing half the unique vaccine and half tweaked to match the BA.4 and BA.5 omicron strains that till lately had been dominant. Now BA.5 descendants are accountable for most COVID-19 circumstances.
The CDC final month launched the primary real-world information displaying that an up to date booster, utilizing both firm’s model, does supply added safety to adults. The evaluation discovered the best profit was in individuals who’d by no means had a previous booster, simply two doses of the unique COVID-19 vaccine — however that even those that’d had a summertime dose had been extra protected than in the event that they’d skipped the most recent shot.



