[ad_1]
It’s a paradox: Life wants water to outlive, however a world filled with water can’t generate the biomolecules that may have been important for youth. Or so researchers thought.
Water is in every single place. Most of the human physique is manufactured from it, a lot of planet Earth is roofed by it, and people can’t survive greater than a couple of days with out consuming it. Water molecules have distinctive traits that permit them to dissolve and transport compounds by means of your physique, present construction to your cells, and regulate your temperature. In truth, the essential chemical reactions that allow life as we all know it require water, photosynthesis being one instance.
However, when the primary biomolecules like proteins and DNA began coming collectively within the early levels of planet Earth, water was really a barrier to life.
The motive why is surprisingly easy: The presence of water prevents chemical compounds from shedding water. Take, for instance, proteins, that are one of many major lessons of organic molecules that make up your physique. Proteins are, in essence, chains of amino acids linked collectively by chemical bonds. These bonds are fashioned by means of a condensation response that ends in the lack of a molecule of water. Essentially, the amino acids must get “dry” as a way to kind a protein.
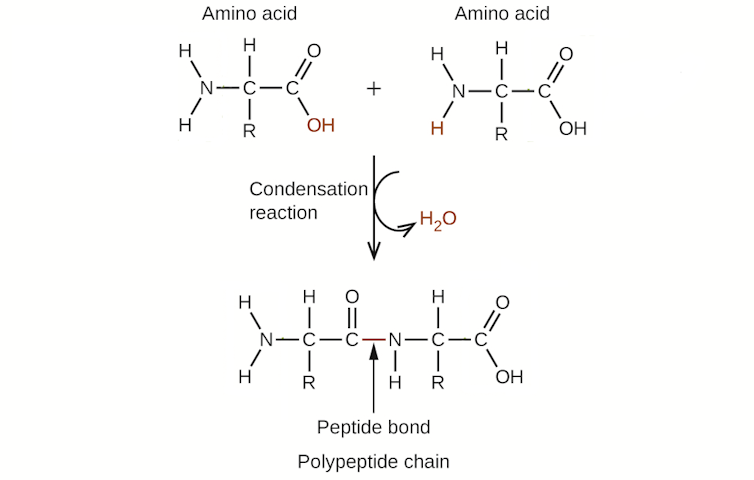
Considering that Earth earlier than life was coated in water, this was a large downside for making the proteins important to life. Like attempting to get dry inside a swimming pool, two amino acids would have had a tough time shedding water to return collectively within the primordial soup of early Earth. And it wasn’t solely proteins that confronted this downside within the presence of water: Other biomolecules important to life, together with DNA and sophisticated sugars, additionally depend on condensation reactions and shedding water to kind.
Over the years, researchers have proposed many options to this “water paradox.” Most of them depend on very particular situations on early Earth that might have allowed water elimination. These embrace drying puddles, mineral surfaces, scorching springs and hydrothermal vents, amongst others. These options, whereas believable, require explicit geological and chemical circumstances that may not have been commonplace.
In our latest examine, my colleagues and I discovered an easier and extra basic answer to the water paradox. Quite sarcastically, it could be water itself—or to be extra exact, very small water droplets—that allowed early biomolecules to kind.
Why Microdroplets?
Water droplets are in every single place, each within the fashionable world and particularly throughout prebiotic (or pre-life) Earth. In a planet coated by crashing waves and raging tides, the small water droplets in sea spray and different aerosols would have plausibly offered a easy and plentiful place for the first biomolecules to assemble.
Water microdroplets—usually very small droplets with diameters round a millionth of a meter, far smaller than the diameter of spider silk—may not appear to resolve the water paradox at first, till you think about the very explicit chemical environments they create.
Microdroplets have a considerable floor area-to-volume ratio that will get bigger the smaller the droplet is. This means there’s a vital area the place the solvent they’re manufactured from (on this case, water) and the medium they’re surrounded by (on this case, air) meet.
Over the years, researchers have proven that the air-water interface is a novel chemical surroundings. The chemistry of those microdroplet interfaces is dominated by giant electrical fields, partial solvation the place molecules are partially surrounded by water, extremely reactive molecules, and very excessive acidity. All these elements permit microdroplets to speed up the chemical reactions that happen in them.
Our lab has been learning microdroplets for a decade, and our earlier work has proven how the speed of frequent chemical reactions could be sped as much as a million occasions sooner in microdroplets. Reactions that may have taken a full day might now be full in only a fraction of a second utilizing these small droplets.
In our latest work, we proposed that microdroplets might be an answer to the water paradox as a result of their air-water interface not solely accelerates reactions but in addition acts as a “drying surface” that facilitates the reactions wanted to create biomolecules regardless of the presence of water.
We examined this concept by spraying amino acids dissolved in microdroplets of water towards a mass spectrometer, an instrument that can be utilized to investigate the merchandise of a chemical response. We discovered that two amino acids can efficiently be a part of collectively within the presence of water through microdroplets. When we added extra amino acids and collided two sprays of this combination collectively, mimicking crashing waves within the prebiotic world, we discovered that this may kind quick peptide chains of as much as six amino acids.
Our findings recommend that water microdroplets in settings like sea spray or atmospheric aerosols have been elementary microreactors in early Earth. In different phrases, microdroplets could have offered a chemical medium that allowed the essential molecules of life to kind from the straightforward, small compounds dissolved within the huge primordial ocean that coated the planet.
Microdroplets Past and Future
The chemistry of microdroplets could be useful in tackling present challenges throughout many scientific fields.
Drug discovery, for instance, requires synthesizing and testing a whole lot of 1000’s of compounds to discover a potential new drug. The energy of microdroplet reactions could be built-in with automation and new instruments to hurry up synthesis charges to multiple response per second in addition to biological evaluation to lower than a second per pattern.
In this fashion, the identical phenomenon that may have aided the origin of the constructing blocks of life billions of years in the past can now assist scientists develop new medicines and supplies sooner and extra effectively.
Perhaps J.R.R. Tolkien was proper when he wrote: “Such is oft the course of deeds that move the wheels of the world: small hands do them because they must, while the eyes of the great are elsewhere.”
I consider the significance of those small droplets is much greater than their tiny measurement.![]()
This article is republished from The Conversation beneath a Creative Commons license. Read the unique article.
